-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
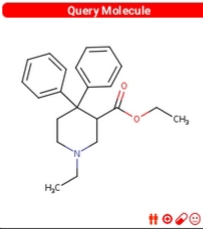
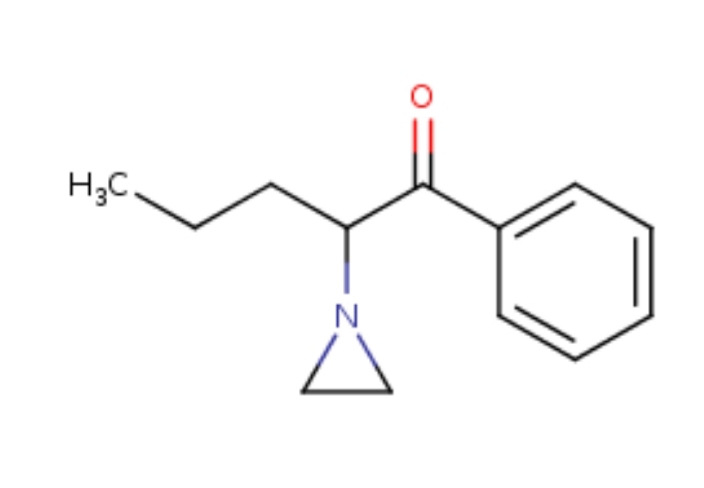
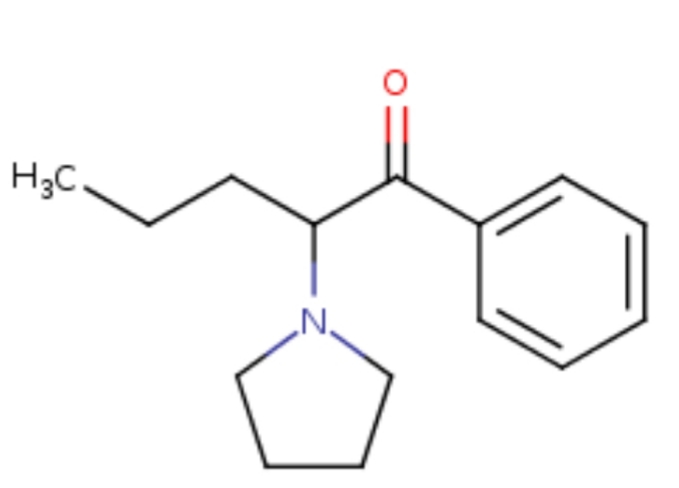
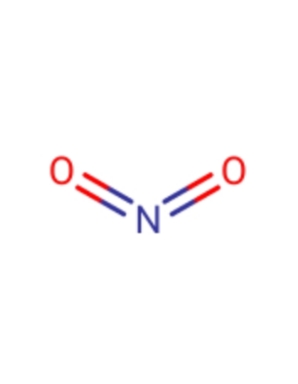
Dresden's Chemical Fluff Thread (Name-A-Molecule)
- Thread starter Dresden
- Start date
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
What do you think it would do?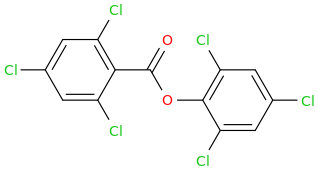
GLOW STICKS
2,4,6-trichlorophenyl-carbonyloxy-2,4,6-trichlorobenzene
See You On The Playa This Coming Labor Day Of Love!!!
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
^ isomeric trichlorobenzoic acids (2,3,6 trichloro) are used as herbicides, acting as auxin analogs.
2,4,6-trichlorophenol is a general biocide (kills everything) that is related to the active ingredient of Dettol antiseptic (chloroxylenol) and also a presumed carcinogen.
2,4,6-trichloroanisole (the methyl ether of 2,4,6, trichlorophenol) is a metabolite of trichlorophenol made in fungus, and has a strongly unpleasant odor. It is found as a flaw in winemaking known as "cork taint".
A closely related compound, the oxalate bis-ester of trichlorophenol, is used as a major component of chemical light sticks, in combination with a fluorescent dye, a weak base, and hydrogen peroxide. That explains the "glow sticks" name.
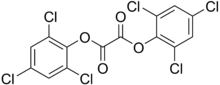
2,4,6-trichlorophenol is a general biocide (kills everything) that is related to the active ingredient of Dettol antiseptic (chloroxylenol) and also a presumed carcinogen.
2,4,6-trichloroanisole (the methyl ether of 2,4,6, trichlorophenol) is a metabolite of trichlorophenol made in fungus, and has a strongly unpleasant odor. It is found as a flaw in winemaking known as "cork taint".
A closely related compound, the oxalate bis-ester of trichlorophenol, is used as a major component of chemical light sticks, in combination with a fluorescent dye, a weak base, and hydrogen peroxide. That explains the "glow sticks" name.

Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
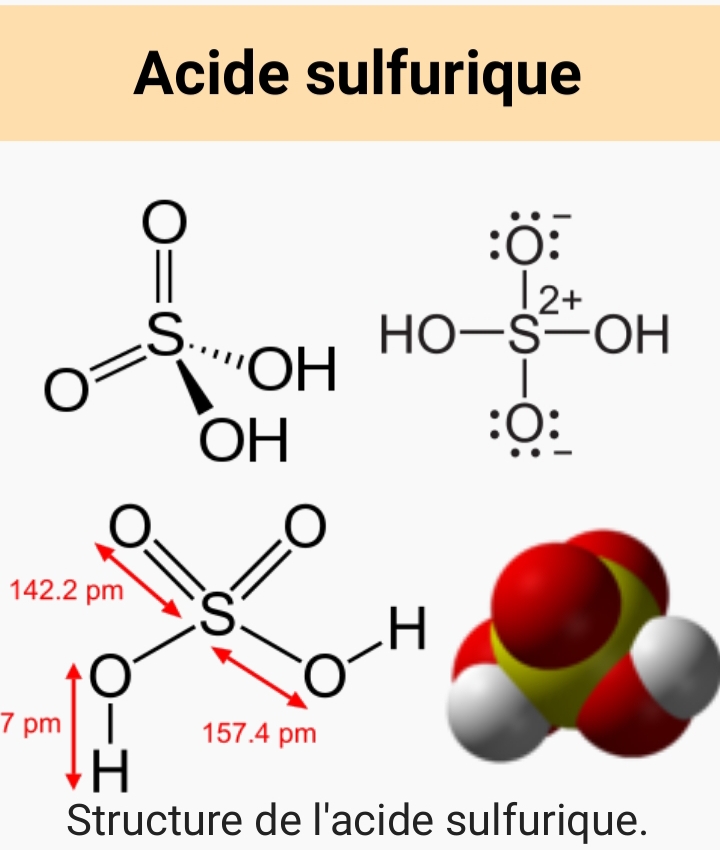
I knew I wasn't wrong!; look!sulfate does not have that structurethat would imply the fully protonated form would be H4SO4.
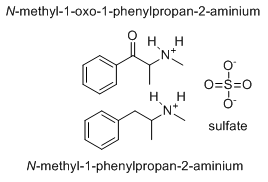
"Methyl-tom and methyl-jerry" ?
polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
That structure describes an electrically neutral sulfuric acid molecule, with the polarity of the chemical bonds shown as the S having a charge of +2 and the oxygens having charge -1. It's probably easy to see only the "+" or "-" charges and think that is supposed to be a structure of a sulfate ion. I think that is the source of confusion here.
polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
What if it is used with R-CNH2 which is neutral, the + should go to the N and the - play its role of acid, like HCL
Here's a picture of amine protonation
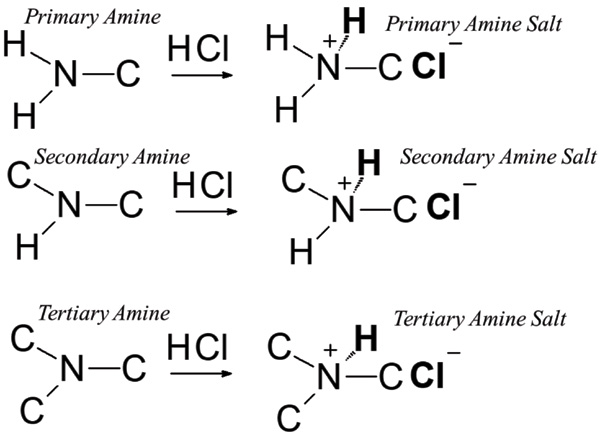
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
So it goes from this:
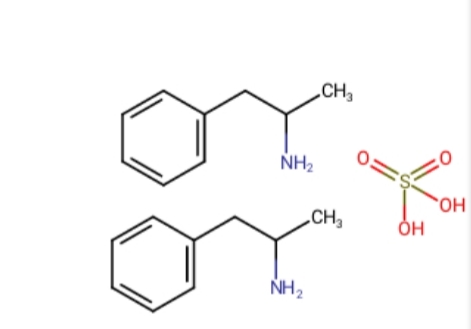
To this:
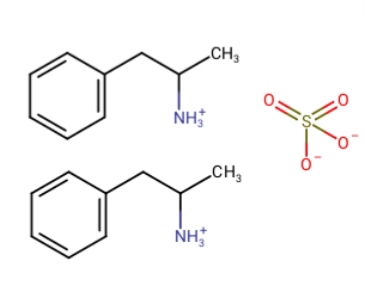
Damn.. I understood wikipedia was wrong when counting hydrogen from the first picture's reaction to this, which is wrong
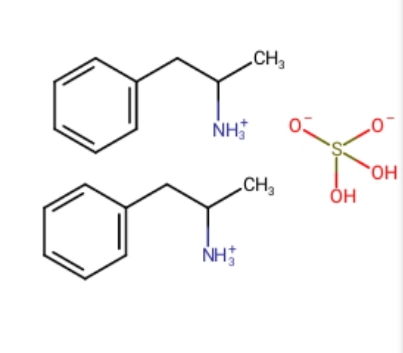
Can a positive charge replace a hydrogen chatge in the third example?
Thus not saying the french wikipedia is wrong? "

To this:

Damn.. I understood wikipedia was wrong when counting hydrogen from the first picture's reaction to this, which is wrong

Can a positive charge replace a hydrogen chatge in the third example?
Thus not saying the french wikipedia is wrong? "
- Joined
- Jul 21, 2002
- Messages
- 12,013