-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Dresden's Chemical Fluff Thread (Name-A-Molecule)
- Thread starter Dresden
- Start date
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
I'LL FEEL'T-TRIP
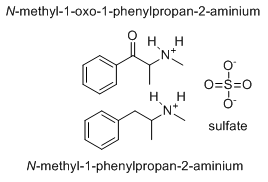
n-monomethyl-ALpha PHenYL'TRYPtamine
SwissTargetPrediction
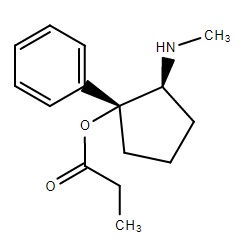
MEOWTWO
SwissTargetPrediction
MEOWTWO GOLD
Last edited:
Has anyone considered that different enantiomers of a chiral compound have different actions. AMT is a good example. When resolved, only 1 isomer has 5HT2a affinity, the other is just stimulating. I suspect that 6,a-DMT would produce entactogenic activity. 6,a,a,-TMT (achiral) is another interesting one.
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
Actually I'm quite tweaked and am trying to figure it out for FACAP, 4F-Phenyl-1-CycloPentyl-(2-Methyleminate) and can't select chirals on my mobile phone on STP. Would you point it out with this given example?Has anyone considered that different enantiomers of a chiral compound have different actions. AMT is a good example. When resolved, only 1 isomer has 5HT2a affinity, the other is just stimulating. I suspect that 6,a-DMT would produce entactogenic activity. 6,a,a,-TMT (achiral) is another interesting one.
Actually I'm quite tweaked and am trying to figure it out for FACAP, 4F-Phenyl-1-CycloPentyl-(2-Methyleminate) and can't select chirals on my mobile phone on STP. Would you point it out with this given example?
STP = DOM? Yes. That would be an excellent example. The example I would really like to test is DON. 2CN is described as 'tofu' and I suspect (R) DON would be similar. You will note that the PEAs are part of the structure of LSD. I HAVE tried the optically resolved AMT but mechanical losses made it expensive. If one was to make 6-methyl AMT, the 6-Me would provide a convenient 'metabolic handle' for the liver to oxidize and thus remove the metabolite. The PEAs & tryptamines overly with the para position of the PEAs overlaying the 6-position of tryptamines. I suggested to Dr. Dave that 3-Me LSD might be interesting and although interested, he baulked at the synthesis.
So often we think about compounds that will never be made due to the complexity of the synthesis. If I mention something, either I HAVE made it or I know someone who HAS made it. I have poor lab technique so I limit myself to working out synthetic pathways. I think it's important to admit one's limitations.
But yeah.... great example. Of course, there are a limited number of synthetic pathways. There IS a supplier in the UK that allows one to obtain the appropriate nitroalkene/nitroalkane so 1 or 2 (2 is still possibly 1 pot) steps.
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
The image isn't showing. ^^
Does this come close to Clubcard's ?:
 swisstargetprediction.ch
swisstargetprediction.ch
It's a carbon and a methoxy away from polymath's
Found it ( I think) :
VIP
Wipped cream, la crème de la crème.
A dedication to Clubcard
5ELO
La MD c'est pour les connards./ DPC
Un clin d'œil ;
A dedication to Sekio
BEASTMODE
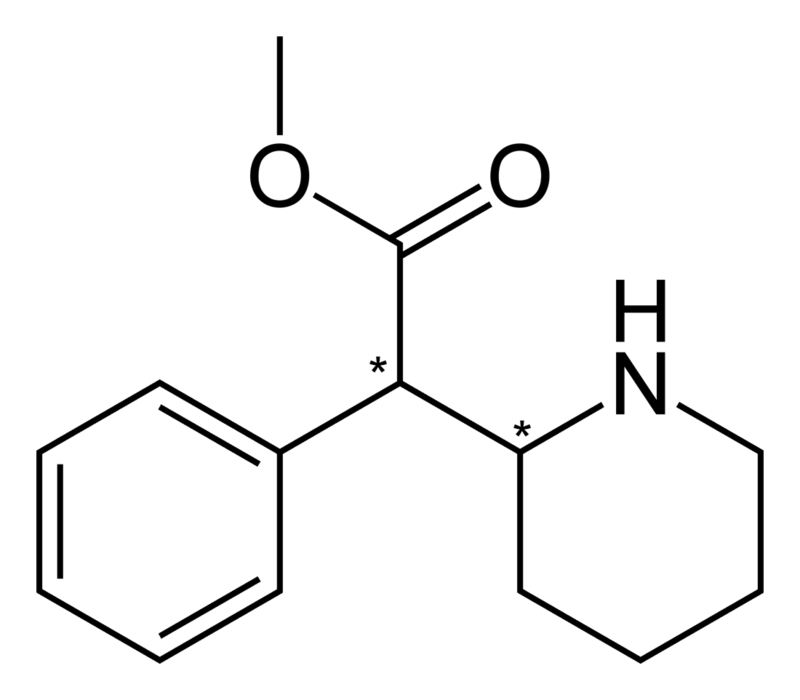
A dedication to Polymath and its funny little beast, Phenmertine
Does this come close to Clubcard's ?:
SwissTargetPrediction
It's a carbon and a methoxy away from polymath's
Found it ( I think) :
VIP
Wipped cream, la crème de la crème.
A dedication to Clubcard
5ELO
La MD c'est pour les connards./ DPC
Un clin d'œil ;
A dedication to Sekio
BEASTMODE
A dedication to Polymath and its funny little beast, Phenmertine
Last edited:
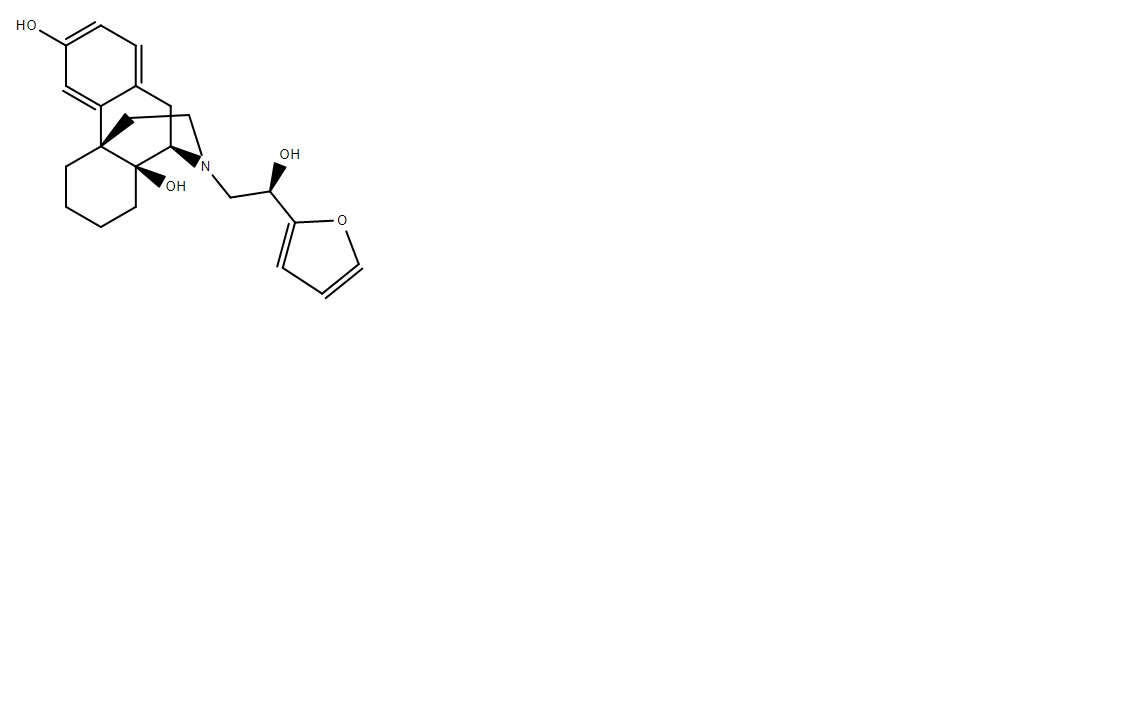
Don't have a name but it's x360 M. If someone can figure out how to add a methyl ether to the 14 then expect that figure to go up a LOT.The TI on it is something like 285. If anyone can figure out how to add a methyl ether to the 14 then it will increase affinity to delta receptors and as well as increasing potency, it will also increase TI. I'm sure people have seen 14-methoxymetopon (x500M) well, check out the TI on that thing. it's truly massive.
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
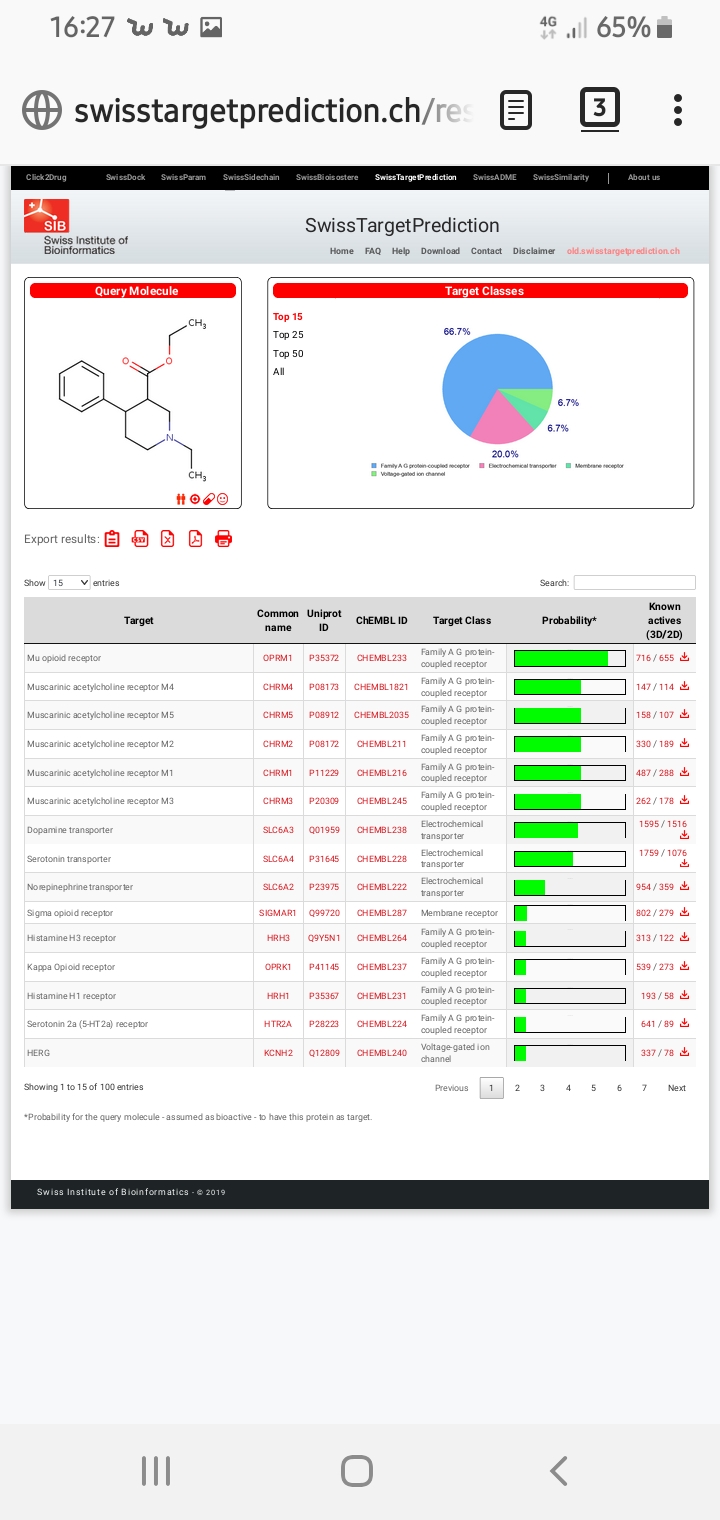
PIPERIPHONE
Ketobemidone's girlfriend, delirious dreams.
EARLY 2000s GERMAN RAVE PILL
Probably the best, usually containing MDMA, AMP, 4MPPP and MDPPP all as a mixture! Those were the good times! (Told to me by a 40 year old raver!) And backed up by papers about MDPPP amd 4MPPP apparitions in Germany in the early 2000s sold along MDMA and AMP in pills.
Last edited:
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
F*CK UP
Because fu them, right?
THE PASSENGER
Because he sure ain't driving
"My Heroin has a passenger.. "
ERASER
You won't even see yourself in the mirror
"IT"
The clown of fentantyls.
Was (is?) being sold as CAR-FU, Carfuranylfentanyl.
Last edited: