alchemist137
Greenlighter
- Joined
- Mar 6, 2019
- Messages
- 11
Planned steps:
Cheers,
ac137
- take 10 x 54 mg Concerta
- put them into 100mL distilled water
- dissolve the outer layer (= some paint, wax and MPH)
- filter out the red paint
- let's call the remaining filtered water+MPH as S1 (for solution 1)
- take the pills out
- cut down the green parts
- put the rest into a coffee grinder (electric) 20k RMP or so
- make fine powder out of the 10 pills using the coffee grinder, let's call this powder as P1
- put the S1 solution into freezer (including the container too, say glass)
and cool both (S1 and the container in which it is) near to freezing point - put P1 into S1 and stir it for a few minutes
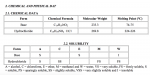
for how long... that is a good question, depends on the solubility of MPH at 5 degree Celsius, below are a few solubility curves.- https://courses.lumenlearning.com/boundless-chemistry/chapter/factors-affecting-solubility/
- https://courses.lumenlearning.com/cheminter/chapter/how-temperature-influences-solubility/
- so 100mL water can dissolve about 2g of MPH at 20-25 degree of Celsius, an educated guess looking at those plots is that it can also dissolve 600mg, so perhaps
200mL water would be a better bet
- If water is cold then most of the Gelatine stays out of the solution, hence the cooling.
- Now filter S1 (which has MPH + some Gelatine and other fillers whatnot in it), let's call the filtered version as S2
- Add n-hexane on top of S2, it should be a separate layer, let's call this two layer "system" as S3, how much n-hexane, that is a good question. I do not know. Here some help would be welcome.
- Add NaOh to S3 , make it a bit alkaline (how much, that is a good question), say there should be twice as many Na ions in the solution than MPH molecules.
- So there will be a point where there will be NaCl (table salt) MPH base and the fillers from the liquid, but no excess Na. But just to be on the safe side, add twice as many NaOh molecules as MPH molecules are in the solution, S3.
- Shake it.
- Remove n-hexane, put it into a new "jar", let's call this solution as S4 (this should contain MPH in base form)
- Add pure water to S4 (water because of the following other solvents are out of question):
- n-hexane is soluble in ethanol, ethyl ether, chloroform
- miscible with alcohol in general
- There is a big question here how much water one really needs here... in principle 10mL is more than enough, which is handy because it evaporates quickly.
- Add HCl to S4 just enough to make the solution a bit acidic, say twice as many molecules as MPH molecules are in S4.
- Remove n-hexane (now this can be tricky without good equipment if there is only 10mL water. But in the worst case one leaves a bit of n-hexane and then heats S4 to 80 degree in a water bath and since the boiling point of n-hexane is 70 degree Celsius, it will boil away and we are left with pure water, a bit of Cl and MPH (in theory).
- Evaporate the water. Let's call what is left as M1 , as in MPH-CL
- Do recrystallisation:
- Dissolve M1 in as little pure as possible IPA at 50 degree Celsius (in water bath)
- or more IPA ? =>I am not sure because I do not have a deep understanding on how two solvent crystallisation works exactly, it might be that a bit more IPA might be better because "chemistry is complicated" ... I don't want to go into deep theories here)
- add n-hexane up to the point when the solution starts to become "cloudy". Let's call the resulting liquid as S5.
- Dissolve M1 in as little pure as possible IPA at 50 degree Celsius (in water bath)
- Put S5 into freezer.
- Collect crystals.
- Put crystals into your vitrine. Next to your other crystal collections, after all, you are just a crystal collector. Some people collect stamps, some people collect crystals.
Cheers,
ac137