aced126
Bluelighter
- Joined
- May 18, 2015
- Messages
- 1,047
https://en.wikipedia.org/wiki/Bupropion#Pharmacodynamics
Hydroxybupropion (HB) reaches plasma concentrations several times greater than bupropion itself, when administered orally. Thus metabolites of bupropion contribute significantly to its mechanism of action. This suggests to me that other N-alkylated-b-keto-halogenated amphetamines could be metabolised in this way and some, if not a majority, of the effects of the drug could be due to this.
Why is bupropion being metabolised this way? Why is the terminal carbon being hydroxylated? We do not see this type of metabolism in amphetamine. Instead, we see ring hydroxylation and benzyl hydroxylation. The first thing that comes to mind obviously is that there are 2 electron withdrawing groups on the ring in bupropion, decreasing ring nucleophilicity. Specifically, though, if the carbon adjacent to the nitrogen wasn't tertiary, would it be hydroxylated instead?
Anyway, with the formation of HB, the alcohol can attack the carbonyl and form phenmetrazine-like hemiacetals, like radafaxine:

These compounds have similar pharmacodynamic properties to bupropion.
Now, would we observe this type of metabolism in similar N-alkylated-b-keto-halogenated amphetamines, like Flephedrone or Clephedrone? To a lesser extent, could this also be observed in non halogenated amphetamines like mephedrone, methcathinone, ethcathinone, and the likes? If so, could they be partly mediating the CNS or peripheral effects of these drugs?
Even if the carbon adjacent to the nitrogen is not tertiary and is hydroxylated in metabolism, could then these 5 membered oxazolidine derivates form and mediate the effects of the drug?
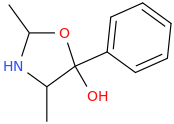
Clephedrone oxazolidine metabolite:

Ethcathinone oxazolidine metabolite:
Would these compounds act as monoamine releasers (e.g would they induce a DAT mediated inward current when exposed to a neuron?).
If my hypothesis is correct that these metabolic compounds are playing significant effects, then it begs the question as to what the following plan oxazolidines/morpholines would do (few examples given here):
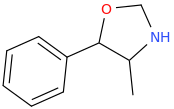
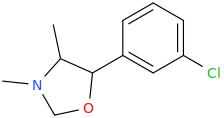
I have almost certainly posted these compounds before, but only now have I realised they might be of more significance than just semi-thought out sketches.
Of course, we have phenmetrazine and derivatives, but they were mainly DAT selective so probably couldn't have any potential as a similar compound to bupropion. Although maybe some slight tweaks to the molecule to make it more NE selective could be a start.
Hydroxybupropion (HB) reaches plasma concentrations several times greater than bupropion itself, when administered orally. Thus metabolites of bupropion contribute significantly to its mechanism of action. This suggests to me that other N-alkylated-b-keto-halogenated amphetamines could be metabolised in this way and some, if not a majority, of the effects of the drug could be due to this.
Why is bupropion being metabolised this way? Why is the terminal carbon being hydroxylated? We do not see this type of metabolism in amphetamine. Instead, we see ring hydroxylation and benzyl hydroxylation. The first thing that comes to mind obviously is that there are 2 electron withdrawing groups on the ring in bupropion, decreasing ring nucleophilicity. Specifically, though, if the carbon adjacent to the nitrogen wasn't tertiary, would it be hydroxylated instead?
Anyway, with the formation of HB, the alcohol can attack the carbonyl and form phenmetrazine-like hemiacetals, like radafaxine:

These compounds have similar pharmacodynamic properties to bupropion.
Now, would we observe this type of metabolism in similar N-alkylated-b-keto-halogenated amphetamines, like Flephedrone or Clephedrone? To a lesser extent, could this also be observed in non halogenated amphetamines like mephedrone, methcathinone, ethcathinone, and the likes? If so, could they be partly mediating the CNS or peripheral effects of these drugs?
Even if the carbon adjacent to the nitrogen is not tertiary and is hydroxylated in metabolism, could then these 5 membered oxazolidine derivates form and mediate the effects of the drug?
Clephedrone oxazolidine metabolite:

Ethcathinone oxazolidine metabolite:

Would these compounds act as monoamine releasers (e.g would they induce a DAT mediated inward current when exposed to a neuron?).
If my hypothesis is correct that these metabolic compounds are playing significant effects, then it begs the question as to what the following plan oxazolidines/morpholines would do (few examples given here):


I have almost certainly posted these compounds before, but only now have I realised they might be of more significance than just semi-thought out sketches.
Of course, we have phenmetrazine and derivatives, but they were mainly DAT selective so probably couldn't have any potential as a similar compound to bupropion. Although maybe some slight tweaks to the molecule to make it more NE selective could be a start.
Last edited:
