mr peabody
Bluelight Crew
- Joined
- Aug 31, 2016
- Messages
- 5,714

Psychedelic treatment approaches to Autoimmune Disorders*
by Joel Ng, MA | Psychedelic Science Review | 11 Dec 2020
There is much research into psychedelic treatment of mental disorders, but psychedelics might be effective in treating autoimmune disorders as well.
After being banned in the United States in 1970, psychedelic compounds have recently made their way back into the attention sphere of the mainstream medical community for their efficacy in treating mental illnesses such as PTSD, depression, and anxiety. Current research into psychedelic drugs primarily focuses on their potential in treating mental illnesses, however, there exists some evidence for their potential in treating another field of illnesses called autoimmune disorders. In this article, the potential link between autoimmune disorders and mental illnesses will be explored, along with possible biochemical pathways that might explain psychedelic’s efficacy in treating both types of conditions.
What are Autoimmune Disorders?
Autoimmunity is when the body’s immune system malfunctions and attacks healthy tissue. The link between autoimmune disorders and mental disorders has received increasing attention in the past twenty years. Being diagnosed with an autoimmune condition increases one’s chances of being diagnosed with a mood disorder. Research into how these two disorders are related has revealed several potential pathways, ranging from chronic inflammation, dysregulation of the hypothalamic-pituitary-adrenal axis (HPA axis), disruptions in the gut microbiome, and chronic stress and trauma. Examining these potential causes, there are many points in which psychedelics might be acting on to explain their treatment efficacy.
Psychedelic Pharmacology
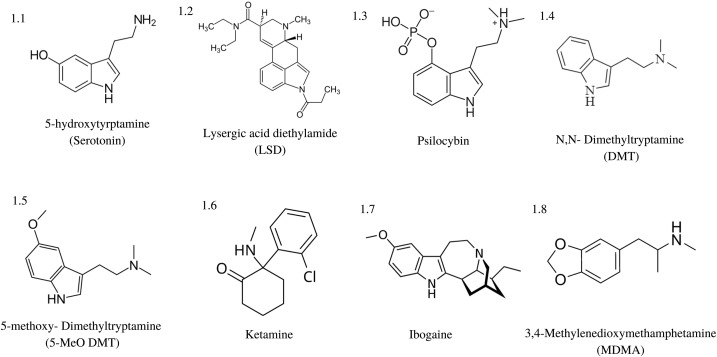
Classic psychedelics exhibit agonistic activity at various serotonin (5-HT) receptors, with the most commonly shared feature being 5-HT2A receptor agonist binding. The table below details some common psychedelics and the receptor subtypes they are known to bind to.
Psychedelic | 5-HT Subtype Affinity | Other Receptor Affinity |
|---|---|---|
LSD | 1A, 1B, 1D, 1E, 2A, 2B, 2C, 5A, 6, and 7 | Dopaminergic receptors subtypes D1 and D2 |
Psilocin | 1A, 1D, 2A, 2C | Adrenergic alpha A and B, dopamine D3, histamine H1 |
DMT | 1A, 1B, 1D, 2A, 2B, 2C, 5A, 6 and 7 | Glutamate receptors, dopamine, acetylcholine, and the opioid-like receptor Sig1R |
Other psychedelic compounds like ketamine affect N-Methyl-D-aspartate (NMDA) receptors as well, which research shows might affect autoimmune disorders via its effect on glutamate pathways.
Inflammation and Immune Modulation
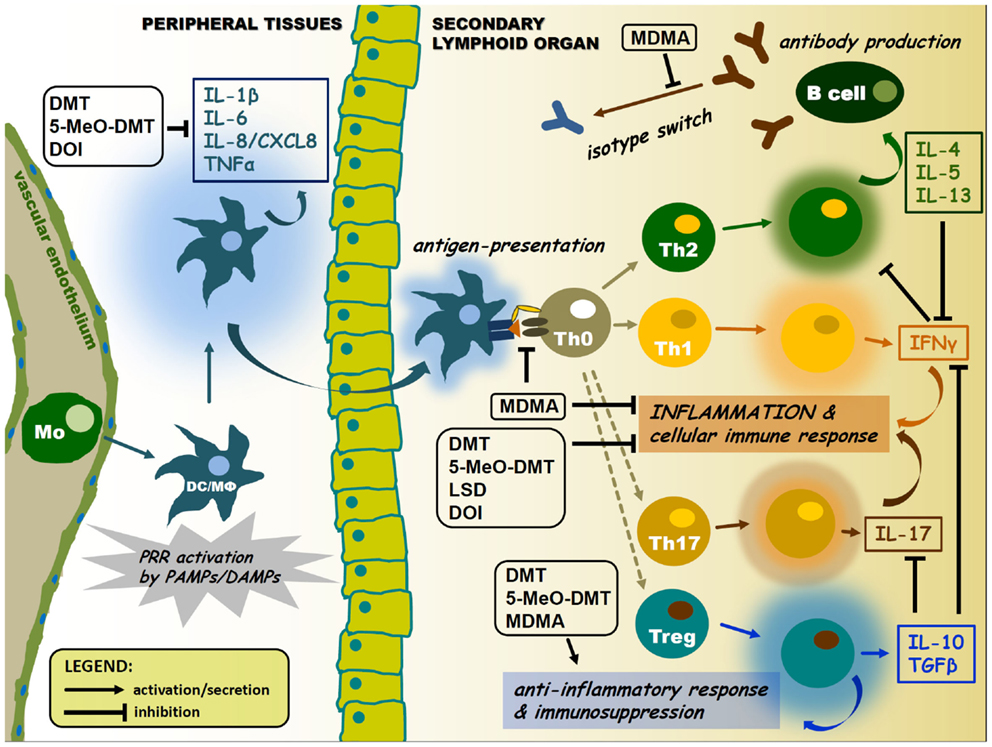
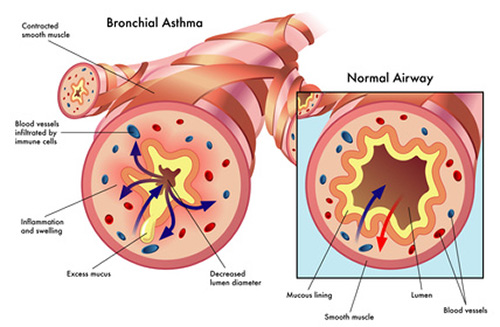
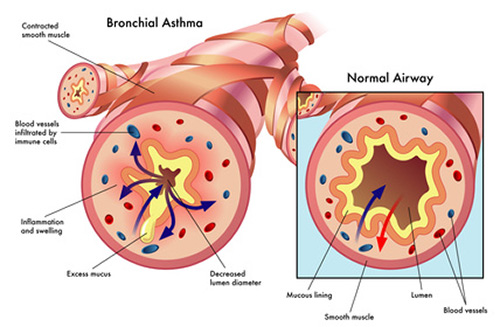
Research has shown that chronic inflammation plays a key role in autoimmune disorders. Dysregulated inflammation is present in many autoimmune disorders, such as Lupus, Rheumatoid Arthritis, Systemic Sclerosis, and Sjögren’s Syndrome. Correspondingly, there has been much research into the 5HT system and its inflammation and immune regulation properties. Sig1R, another receptor that psychedelics like DMT effect, also modulates inflammatory and immune responses. Via their effect on the 5HT system and Sig1R system, psychedelics might have anti-inflammatory properties that could explain their potential in treating autoimmune disorders.
Trauma and Stress
Another pathway where mental disorders and autoimmune disorders might be linked lies in stressful and traumatic childhood events. Adverse childhood experiences (ACEs) are strongly correlated with autoimmune disorders in later life. Research into the effect that psychological stress has on the immune system reveals that psychological stress can compromise the immune system function as well. This leads to disorders such as food sensitivity, HPA axis dysregulation, and leaky gut syndrome, all of which are strongly correlated with autoimmune disorders. This research hints at how psychedelic treatment of trauma-related illnesses might also improve physiological ailments related to trauma.
Glutamate and Brain-Derived Neurotrophic Factor
Glutamate excitotoxicity involves overactive glutamate receptors in the brain. Chronic excitation of glutamate receptors can contribute to oxidative stress, causing brain damage. Glutamate excitotoxicity is related to depression, addiction, and other neurodegenerative diseases. Examining ketamine’s effect on NMDA glutamate receptors, it is possible that ketamine’s effect on depression might be related to glutamate regulation. Agonists of the Sig1R receptor subtype have also shown to be neuroprotective glutamate excitotoxicity. Also, DMT has been shown to have potent neuroprotective effects via the Sig1R.
Psychedelics might increase levels of brain-derived neurotrophic factor (BDNF) gene expression, which facilitates neurogenesis. Low BDNF expression has been linked to depression, anxiety, schizophrenia, and neurodegenerative diseases. This pathway is related to 5-HT2A receptor agonist activity of classic psychedelics – in an experiment, administration of a 5-HT2A antagonist reduced the neuroplastic effects of classic psychedelics LSD, DMT, and DOI. It is feasible that the BDNF-upregulating qualities of psychedelics could reduce the inflammatory effects of glutamate excitotoxicity in autoimmune disease.
Gut Microbiome Dysregulation
The gut microbiome refers to the microorganisms present in the gastrointestinal tract (GIT). The gut microbiome produces many neurotransmitters in the body, including dopamine, serotonin, norepinephrine, and gamma-aminobutyric acid (GABA). Chronic infections with bacteria, fungi, and viruses are common in those with autoimmune disorders. Microbiome bacterial populations appear to play a significant role in a number of immune responses, and influence the presence of autoimmune disorders.
Psychedelic plants like ayahuasca and peyote have been shown to exhibit anti-microbial properties, which may explain how psychedelics can effectively treat autoimmune conditions. Also, experiments that introduced serotonin to the gut microbiome indicate the presence of a bidirectional signalling system between the host serotonin system and the gut microbiome. This highlights the potential for psychedelic serotonin analogues psilocybin, DMT, and 5-MeO-DMT interacting with bacterial receptors.
Lastly, the psychological benefits of psychedelics may indirectly alter microbial communities in the GIT via induced changes in vagal nerve tone and stress response. Several studies examining the effects of chronic stress and PTSD on microbiome pattern associations found a link between stress and microbiome health. The psychological and neurological benefits from a psychedelic experience may cause biochemical cascades in the HPA axis and the nervous system, which may influence the ecology of microbial populations.
Conclusion
Autoimmune disorders involve a malfunction of the body’s immune system, causing it to attack the body it is meant to protect. While the causes of autoimmune diseases are varied, several pathways have been proposed involving neurotransmitter dysfunction, gut microbiome dysfunction, and an assortment of other dysregulations present in the nervous system’s biochemistry.
There appears to be an immensely complex biochemical network involving the peripheral nervous system, the gut microbiome, and the immune system. This helps explain psychedelics’ well-known efficacy in treating mental illnesses and also highlights the potential for psychedelics to treat autoimmune disorders.
Further research into the potential biochemical pathways that connect these areas of the body may provide more effective psychedelic treatment programs for mental illnesses. Also, these studies lay the groundwork for using psychedelics for treating of a whole other class of disorders involving the immune system.
*From the article here :

 psychedelicreview.com
psychedelicreview.com
Inflammation and Immune Modulation
Research has shown that chronic inflammation plays a key role in autoimmune disorders. Dysregulated inflammation is present in many autoimmune disorders, such as Lupus, Rheumatoid Arthritis, Systemic Sclerosis, and Sjögren’s Syndrome. Correspondingly, there has been much research into the 5HT system and its inflammation and immune regulation properties. Sig1R, another receptor that psychedelics like DMT effect, also modulates inflammatory and immune responses. Via their effect on the 5HT system and Sig1R system, psychedelics might have anti-inflammatory properties that could explain their potential in treating autoimmune disorders.
Trauma and Stress
Another pathway where mental disorders and autoimmune disorders might be linked lies in stressful and traumatic childhood events. Adverse childhood experiences (ACEs) are strongly correlated with autoimmune disorders in later life. Research into the effect that psychological stress has on the immune system reveals that psychological stress can compromise the immune system function as well. This leads to disorders such as food sensitivity, HPA axis dysregulation, and leaky gut syndrome, all of which are strongly correlated with autoimmune disorders. This research hints at how psychedelic treatment of trauma-related illnesses might also improve physiological ailments related to trauma.
Glutamate and Brain-Derived Neurotrophic Factor
Glutamate excitotoxicity involves overactive glutamate receptors in the brain. Chronic excitation of glutamate receptors can contribute to oxidative stress, causing brain damage. Glutamate excitotoxicity is related to depression, addiction, and other neurodegenerative diseases. Examining ketamine’s effect on NMDA glutamate receptors, it is possible that ketamine’s effect on depression might be related to glutamate regulation. Agonists of the Sig1R receptor subtype have also shown to be neuroprotective glutamate excitotoxicity. Also, DMT has been shown to have potent neuroprotective effects via the Sig1R.
Psychedelics might increase levels of brain-derived neurotrophic factor (BDNF) gene expression, which facilitates neurogenesis. Low BDNF expression has been linked to depression, anxiety, schizophrenia, and neurodegenerative diseases. This pathway is related to 5-HT2A receptor agonist activity of classic psychedelics – in an experiment, administration of a 5-HT2A antagonist reduced the neuroplastic effects of classic psychedelics LSD, DMT, and DOI. It is feasible that the BDNF-upregulating qualities of psychedelics could reduce the inflammatory effects of glutamate excitotoxicity in autoimmune disease.
Gut Microbiome Dysregulation
The gut microbiome refers to the microorganisms present in the gastrointestinal tract (GIT). The gut microbiome produces many neurotransmitters in the body, including dopamine, serotonin, norepinephrine, and gamma-aminobutyric acid (GABA). Chronic infections with bacteria, fungi, and viruses are common in those with autoimmune disorders. Microbiome bacterial populations appear to play a significant role in a number of immune responses, and influence the presence of autoimmune disorders.
Psychedelic plants like ayahuasca and peyote have been shown to exhibit anti-microbial properties, which may explain how psychedelics can effectively treat autoimmune conditions. Also, experiments that introduced serotonin to the gut microbiome indicate the presence of a bidirectional signalling system between the host serotonin system and the gut microbiome. This highlights the potential for psychedelic serotonin analogues psilocybin, DMT, and 5-MeO-DMT interacting with bacterial receptors.
Lastly, the psychological benefits of psychedelics may indirectly alter microbial communities in the GIT via induced changes in vagal nerve tone and stress response. Several studies examining the effects of chronic stress and PTSD on microbiome pattern associations found a link between stress and microbiome health. The psychological and neurological benefits from a psychedelic experience may cause biochemical cascades in the HPA axis and the nervous system, which may influence the ecology of microbial populations.
Conclusion
Autoimmune disorders involve a malfunction of the body’s immune system, causing it to attack the body it is meant to protect. While the causes of autoimmune diseases are varied, several pathways have been proposed involving neurotransmitter dysfunction, gut microbiome dysfunction, and an assortment of other dysregulations present in the nervous system’s biochemistry.
There appears to be an immensely complex biochemical network involving the peripheral nervous system, the gut microbiome, and the immune system. This helps explain psychedelics’ well-known efficacy in treating mental illnesses and also highlights the potential for psychedelics to treat autoimmune disorders.
Further research into the potential biochemical pathways that connect these areas of the body may provide more effective psychedelic treatment programs for mental illnesses. Also, these studies lay the groundwork for using psychedelics for treating of a whole other class of disorders involving the immune system.
*From the article here :

It Takes Guts: Psychedelic Treatment Approaches to Autoimmune Disorders
There is much research into psychedelic treatment of mental disorders, but psychedelics might be effective in treating autoimmune disorders as well.
Last edited: