'Patients say ayahuasca is like a reboot for the brain'
by Ayelett Shani Aug 30, 2018
The reason psychoactive drugs are banned is strictly cultural, says neurobiologist Dr. Ido Magen from the Weizmann Institute of Science. We need to free ourselves from the dichotomy between drugs and medications
What is it with you and drugs?
I did my doctorate under Prof. Raphael Meshulam on the subject of cannabis. The truth is that it’s not something that I was aiming for. It turned out that I got to a lab that happened to be doing a study with Meshulam, who is the high priest of cannabis worldwide. The idea was to use substances that he synthesizes from cannabis to treat a liver disease. We used CBD, which is the non-psychoactive element in cannabis, and saw that it actually worked.
Back when I was working on my Ph.D., I asked myself how it was possible that alcohol is legal and cannabis isn’t. That really bugged me. I knew from the research that it’s not a dangerous substance. As I extended my knowledge on the subject, I saw that the source of the ban on cannabis is actually cultural: racism toward Hispanics who immigrated to the United States in the 1930s. My interest only deepened. I began to collect material systematically, and at some point I started to lecture on drugs and to write articles. Somehow it gathered momentum.
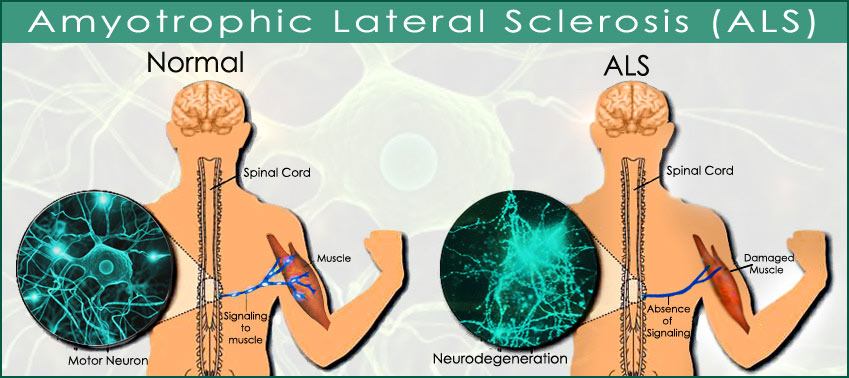
Today, in my day-to-day work I am investigating the disease ALS [Lou Gehrig’s Disease] from completely different directions, but I continue to take an interest in psychoactive drugs and to lecture and write on the subject.
You are also active publicly on the subject. I saw an extremely sharp letter that you sent to the Israel Anti-Drug Authority.
As someone who deals with the subject from the scientific angle, I consider it absolutely a mission to illuminate the issue from that perspective and perhaps also to uproot the ignorance that characterizes the authorities’ entire attitude toward drugs. I really don’t like the messages that the IADA transmits to the broad public. I don’t like the fact that the state authorities are lying, simply disseminating fake news – or presenting alternative facts, to put it more mildly.
You are critical of the “gateway theory” – cannabis serving as the entryway to hard drugs, the joint that morphs into a syringe, etc.
The IADA claims that there is scientific proof that cannabis leads to the use of hard drugs down the line. That is a total lie. Even the authority itself makes opposite claims on its web page. It presents many figures as being factual, even though they have no empirical basis. For example, that a chronic use of cannabis leads to addiction and that the attempt to stop smoking it involves rehab with harsh physical symptoms; or data about the physical damage that smoking weed entails, among them a heightened risk of fatal diseases, damage to the respiratory system and so forth – whereas there are conflicting and not unequivocal findings about all those effects.
I think that the messages should be accurate and based on facts. In this era, in which people are exposed to a great deal of information, it’s anachronistic, not to say ridiculous, to continue to disseminate messages that are clearly far from the truth.
Many influential people are meddling in the legalization issue.
I think that certain enforcement agencies have an interest in continuing this line, because it’s easier to deal with people who smoke joints than with real criminals. They get very big budgets – the police, after all, are even against decriminalization, although they have no grounds for this other than the excuse that “this is how we catch dealers.” Do people who smoke have to be hostages of the police in order to catch drug dealers? Is the arrest of drug dealers useful in reducing the dimensions of the phenomenon?
What about the pharmaceutical companies?
I don’t have a smoking gun, of course, but there are hypotheses that the pharma companies are behind the super-slow progress being made in regard to medical cannabis. Its export has been bogged down for a few years [in Israel], and the prevailing conjecture, which to me sounds logical, is that the pharmaceutical corporations are behind this. Their interest is clear: They don’t want people to start using cannabis and then reduce or stop taking medications. So I see it as a mission: to free the public of its ignorance and to apprise it of the true facts.
There’s a petition to the court now, submitted by Green Leaf [a political party that advocates the legalization of cannabis] against the criminalization of marijuana, on the grounds that it violates the Basic Law on Human Dignity and Freedom. I was asked to write a professional opinion, and am doing so. It will be part of the petition.
What do you plan to say?
Naturally, I can’t reveal too much, but overall it’s about refuting mini-myths related to cannabis in order to persuade the justices that it’s not some monstrous substance that will lead to a disaster if the ban on its use is lifted.
Drugs and immigrants
Let’s talk about the process, which you address in your talks: namely, the gradual use of recreational drugs in medical treatment. You have a certain perspective on this, because the research you conducted ended a decade ago.
At that time people didn’t talk about cannabis like they do today. There were occasional experiments. Attempts were made to use cannabis in treating anorexia, for example, but it didn’t gain momentum. Today, drugs like these are used to treat a great many diseases and phenomena, after many years during which drugs were considered anathema. In the United States, the process of outlawing drugs began during Prohibition. Alcohol, after all, is a psychoactive drug in every respect, and that law was a reaction to massive immigration from Europe to the United States and to the culture of drinking in Europe, which the authorities very much did not like.
It’s interesting, by the way, that precisely at the stage when the prohibition on alcohol was lifted, the ban on cannabis began. The hypothesis is that the personnel who were in charge of enforcing the alcohol ban were left with no work to do, so they were sent to persecute cannabis users. Until the early 20th century, cannabis was legal and was even sold in drugstores. But when it arrived in the United States together with a wave of immigration from Mexico, it was outlawed. And it’s clear that economic interests were involved then, too.
What about psychedelic drugs?
Psychedelic drugs were very popular in South America and gradually penetrated the United States and the Western world overall. Aldous Huxley tried mescaline, and Timothy Leary, a professor [of psychology] at Harvard, conducted his famous experiment and aroused the fear and suspicion of the establishment. Leary’s experiment was halted and he became the darling of the hippies and of various proponents of the counterculture, who used LSD and mushrooms to the point where they became the very trademarks of the counterculture. Finally, LSD and the mushrooms were outlawed, which didn’t, of course, stop people from continuing to use them.
In our time we’re seeing the opposite trend: recognition of their medical qualities. Why is this so? Why now? Is the approach “Let’s try all existing substances until we find something”?
I can’t pinpoint the exact turning point when this trend started, but it certainly has to do with the discovery of the mechanisms of these drugs and their modes of operation. With cannabis, for example, until Prof. Meshulam discovered its active substances, no one knew what that it did, and that opened a window to a whole world.
In other words, the new conception derives from progress in brain research.
When it was discovered that psychedelic drugs work by activating serotonin, it was already known that illnesses such as depression are related to serotonin. So someone did the calculation that they might be useful for depression, too, but that really is progress made in the past few years. The psychedelic drugs specifically were like outcasts. They were feared because they generate hallucinations, and because most of them are synthetic. The objections to them were greater than for cannabis.
Let’s talk a bit about neural conduction. Roughly and simplistically, information in the brain is transmitted when one cell fires a chemical substance – a neurotransmitter – at another cell. It’s in that encounter, in the space between the cells in which the substance exists, that the influence of drugs occurs.
I like the analogy by which the chemical substance, the neurotransmitter, is like a key, and the receptor to which it attaches is a lock. Each key actually fits one specific lock, with the exception of a few substances that can attach to a number of receptors. Opening the lock causes electrical changes in the door that’s being opened. The drug can work on a particular neurotransmitter, it can inhibit the process of reabsorption into the cell that fired it, as in psychiatric medications; and cocaine and Ecstasy actually reverse the direction of the carrier, the substance that is responsible for returning the neurotransmitter to the cell that released it, so that instead of returning to the cell from which it was fired, it flows to the receiving cell, and thus its effect is prolonged.
The psychoactive drugs intervene in the process of neural conduction in the brain. They can alter it, intensify it or weaken it, extend or abbreviate its duration. Because neural conduction has a direct connection to our feelings, our consciousness, our cognitive functioning, it influences us, as well.
Reward and addiction
What about the reward system in the brain?
The reward system in the brain is actually intended to strengthen natural behaviors that are beneficial to our survival, such as sexual relations, which are critical for the species’ survival, or [a craving for] sweet foods that supply calories. All psychoactive drugs affect the brain’s reward system to one degree or another. Effectively, they deceive the system: We feel that we have done something that benefits us, even though in practice, of course, that’s not true. People use drugs to activate the mechanism by force, simply because that activation gives us pleasure even though it doesn’t serve any survival goal. The drugs trigger the reward mechanism – it signals us that we feel pleasure in the wake of the use of a drug, so we want to repeat the action over and over. That’s addiction.
So the question is not why to use drugs to try to gain relief or to cure mental problems, but why it took so long to arrive at this solution.
The answer is: cultural obstacles and vested interests. Conservatism. Across history, strange as it may sound, psychoactive drugs were not reviled. The ban is relatively new. The length of time in which cannabis, for example, has been prohibited is a tiny dot in our history, because there is evidence of its use going back thousands of years. All told, we’re talking about a hundred years, even less, since it’s been outlawed, but we’re brainwashed into thinking that this is natural.
And in contrast, psychiatric medications are considered totally legitimate and normative, even though in many ways their effect and the way they work are similar.
It’s only a matter of labeling. What’s the difference? A psychiatric medication is a chemical substance that affects the nervous system, and cannabis is a natural substance that affects the nervous system. In English the word “drug” also means medication – there’s no difference in the terminology. In Hebrew there’s a clear distinction [between sahm (drug) and terufah (medication)].
One of things I try to emphasize in my talks is that we need to free ourselves from the dichotomous division between drug and medication and from the mistaken working assumption that a medication is something good and a drug is something bad. All these substances affect our body in one form or another, they affect our functioning and our consciousness.
And in both cases we don’t know the full scope and consequences.
Correct. Opiod medications in the United States are simply killing thousands of people today. It’s ridiculous to think that they should be legal while cannabis isn’t. It’s really only a matter of convention.
Let’s turn to the use of psychedelic drugs for treatment of psychiatric problems. For example, ayahuasca, which has been found effective in the treatment of depression.
Ayahuasca is a herbal brew that was used by Native Americans for ritual purposes. It contains two relevant active substances. One works on serotonin receptors, the second effectively inhibits the breaking-down, thanks to which it’s possible to achieve a prolonged effect of the substance, after we drink it.
We should explain that the drinking of ayahuasca is a ceremony performed under the guidance of a shaman. I know a few people who have tried it. They described a psychedelic experience, hallucinations.
Yes. Obviously, the well-known ceremony was not performed as part of the clinical experiment that was done in a Brazilian university. The subjects, all of whom were suffering from depression and had not been helped by conventional medication, received either ayahuasca or a placebo under the supervision of the researchers. The experiment found that ayahuasca reduced the depressive symptoms, whereas the patients who received the placebo didn’t report any improvement – in fact, in some patients the depressive symptoms became even more acute.
But what was really interesting in that study was that a correlation was found between the intensity of the mystical experience, which was quantified through all kinds of questionnaires, and the improvement in the patients’ condition. The more powerful their experience, the more the depressive symptoms were weakened. That’s interesting, because in another study too, one that used psilocybin, which is produced by magic mushrooms, a connection was found between the intensity of the mystical experience and the level in the blood of a substance that is a product of the breakup of nicotine. In other words, the stronger the mystical experience, the less people smoked.
So there is a correlation between the intensity of the mystical experience and the therapeutic effect.
The conjecture is that the mechanism that causes the mystical sensations is also responsible for relief in the depressive symptoms. Of course, it’s impossible to prove the existence of a causal relationship.
Yes, but it can be assumed that there’s a connection to the release from the shackles of consciousness. The question arises of the degree to which consciousness creates the pathology.
These substances cause a blurring of the senses, liberation. They open the doors of perception, in the words of Aldous Huxley, and that allows us to be exposed to things and content that had been simply hidden from us. Brain scans done on people under the influence of LSD show that their whole brain lights up. There is very powerful activity, a great many regions in the brain are communicating with one another, the sensory experience changes.
Studies on LSD found that one of its effects is to dissolve the sense of the self – a Buddhist truth for liberation from suffering.
It’s not by chance that psychedelic drugs help in the treatment of mental illnesses more than other drugs. Cannabis also affects the physiological system, but with psychedelic drugs the effect on the body is minor; their primary influence is on the brain and on consciousness. Psychedelic drugs will not be used to relieve heart disease, say.
On the other hand, the description of heightened brain activity and a change in the sensory experience sounds similar to what happens in a psychotic attack.
I suppose that’s true – people undergoing a psychotic attack have powerful experiences, hear voices, see colors. That raises the question of whether psychedelic drugs are likely to aggravate schizophrenia or ease it. In any event, they are not used in cases of psychoses. Depression, in contrast, is not a psychosis, and psychedelic drugs can be used in an attempt to treat it. A study conducted in England tried to treat people suffering from depression that is immune to medications by means of psilocybin. The subjects underwent three brain scans in the experiment and immediately after the mushroom treatment. All of them reported a relief of the depression. The scans showed a change in brain activity.
That experiment examined long-term influences. How is it possible that it relieved the symptoms over time? After all, psychiatric drugs need to be taken daily.
That’s an interesting question, to which I don’t think they have an answer. We can only surmise. I find it hard to believe that the use of a drug caused a change in the brain chemistry, in the neurotransmitters. Possibly it’s a matter of dosage. The patients reported that it worked on them like a reboot of the brain. Despite the positive results, it was a preliminary study with a small number of patients, and we have to remember that this substance has side effects, so I wouldn’t recommend taking it just for the fun of it, and certainly if anyone wants to use it to relieve depression, that has to be done under medical supervision.
It’s interesting whether the patients themselves were able to overcome their prejudices about psychedelic drugs. It seems to me that when they’re used, the expectations shape the experience.
In indigenous cultures the psychedelic drugs are perceived as legitimate and are used in ceremonies and so forth. Western culture views them as something foreign, because they come from a foreign culture, and Western culture is not tolerant of anything that is “other.” Consider cannabis, for example, which was in widespread use in pre-Mandate Palestine, but under the British was added to the Dangerous Drugs Ordinance. Why? In my opinion, because they wanted to make it clear that they intended to impose a different culture here.
And the natives here are “drinking hashish” [reference to a line from the 1978 film “Lemon Popsicle”].
Yes. When the Spaniards arrived in South America they also banned the use of psychedelic substances, and for the very same reason. It’s something deeper and bigger than the approach to drugs as such – it’s an approach to what is different from you, something that doesn’t exist in your culture. The very definition of certain substances as “drugs” is a matter of culture. What is alcohol? Isn’t it a drug? Isn’t nicotine a drug? The word “drug” is used to indicate something that is foreign, not to say threatening, to our culture.
https://www.haaretz.com/israel-news...asca-is-like-a-reboot-for-the-brain-1.6431971