UPDATE Anybody who's interested in the topic of morning glory seeds and ergot should read the following post in this thread (#95); and I've made many of my reference on this topic available here: http://www.bluelight.org/vb/entries/7141-morning-glory-seed-information-archive
My latest contributions to this topic can be found in my Shroomery journal: http://www.shroomery.org/forums/postlist.php/Journal/240779
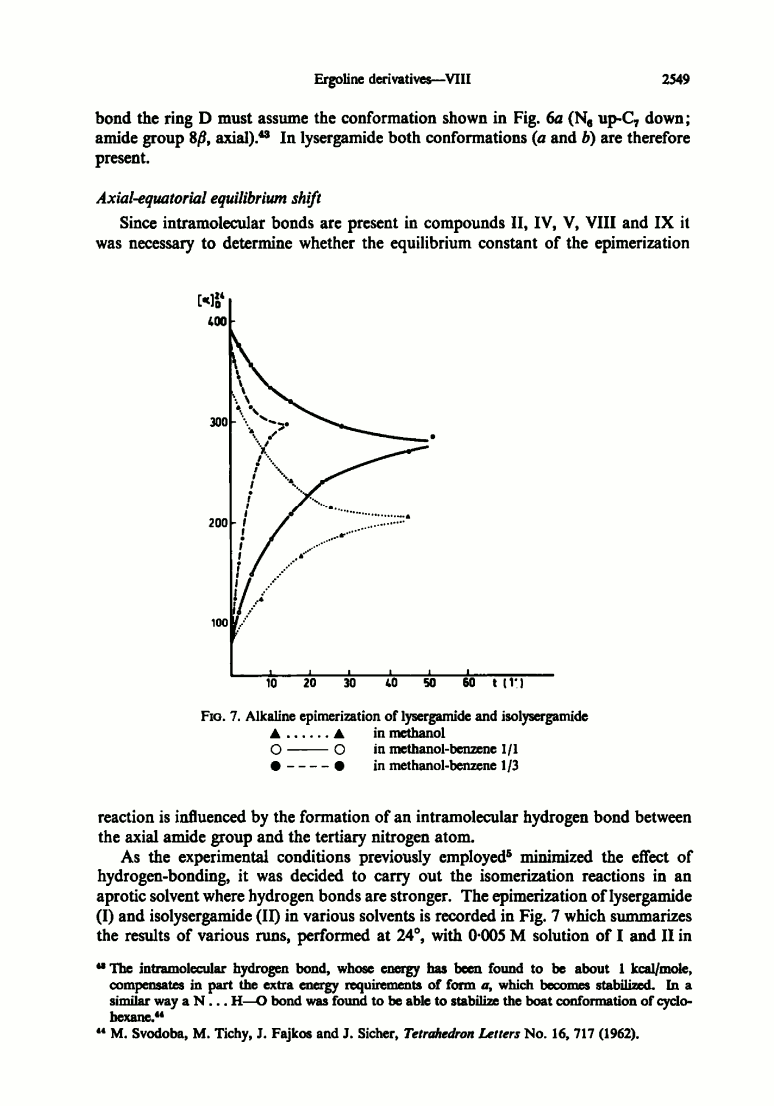
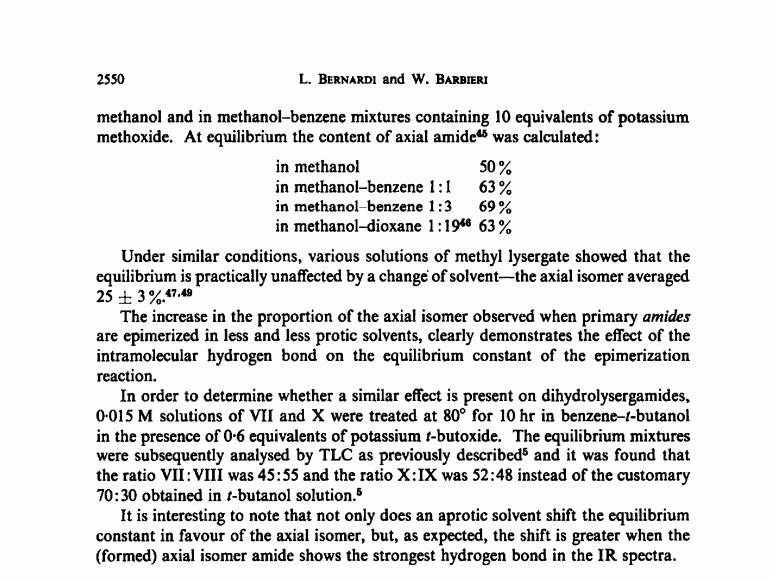
Originally I created this thread to ask about the hypothetical equilibrium of three different forms of ergine that Peter Webster talks about here:
The equilibrium reaction takes some time to occur, perhaps an hour or more, and is brought about most effectively by basic conditions
Peter Webster, "Secret Recipes - Hofmann Symposium," The Invisible College Magazine, issue no. 2, page 30 http://www.earthrites.org/invisible-college.htm
Webster's hypothesis is based on the following paper:
Full paper: http://rapidgator.net/file/64513007/Tetrahedron.pdf.html
My latest contributions to this topic can be found in my Shroomery journal: http://www.shroomery.org/forums/postlist.php/Journal/240779
Originally I created this thread to ask about the hypothetical equilibrium of three different forms of ergine that Peter Webster talks about here:
The equilibrium reaction takes some time to occur, perhaps an hour or more, and is brought about most effectively by basic conditions
Peter Webster, "Secret Recipes - Hofmann Symposium," The Invisible College Magazine, issue no. 2, page 30 http://www.earthrites.org/invisible-college.htm
Webster's hypothesis is based on the following paper:


Full paper: http://rapidgator.net/file/64513007/Tetrahedron.pdf.html
Last edited:


