paracelsius
Bluelighter
- Joined
- Mar 11, 2020
- Messages
- 197
This is an interesting compound I've come across: 4-aza-desoxypipradol (2-diphenylmethyl-piperazine aka 4-azapradol).
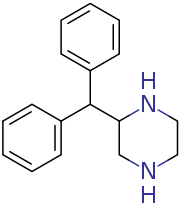
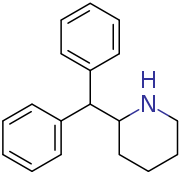
apparently being sold as dopaminergic nootropic stimulant: It is the piperazine analog of desoxypipradol (2-DPMP, 2-diphenylmethyl-piperidine aka Ivory wave) with the piperidine replaced by a piperazine.
It was patented in a old Dutch patent as a CNS stimulant. Can't pull that one out but I am sure I've seen it some years ago (would appreciate a link if anybody came across, thx).
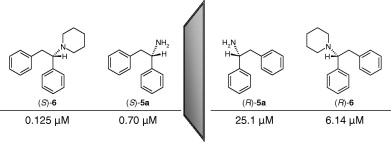
Apparently, it is more potent than 2-DPMP as a NDRI and a cocnaine/MPH-like susbtrate but not AMPH-like inhibitor. Now, 2-DPMP is already one of the most potent NDRI iirc (active starting at 5mg-ish!). That means this compound may be active at micrograms range?!.. One advantage tho it may have shorter half-life than the insane 24h+ of 2-DPMP since the piperazine will decrease the logP by at least 2 log units relaltive to the parent piperidine! making it more polar and more easily excreted. Probably similar DOA to benzylpiperazine BZP (5-7 hours). Lower logP will also make it faster acting compared to highly lipophilic 2-DPMP that tend to accumate in fat and release slowy giving it slower absorption rate, longer half-life and irregular bioavailability. Plus the extra 4-nitrogen may make a nice metabolic handle for conjugation unlike with 2-DPMP or morphodrol (4-oxo-desoxypipradol, morpholine replacing the piperidine)... but I dont know until I try it!
Now, you might wonder why would that one be different from morphodrol which was a bust, had a pretty bad review at least with the nootropics crowd iirc? I don't know. But at least with the DAT transporter it seems a H-bond donor (not acceptor! like with morphodrol Oxygen) increases binding (relative to no H-bond group like in 2-DPMP) and a donor decrease it (like in morphodrol), but I am sure iirc the dutch patent describe it as potent CNS stimulant.. anyway, that is an interesting compound. Now, in that old dutch patent, they make it via pto2 reduction of 2-diphenylmethylpyrazine which itself was made from diphenylacetonitrile and 2-chloropyrazine. Pretty expensive route. Isnt other ways to make it? maybe in 2-step/1-pot from cheap diphenylacetonitrile and piperazine-2-carboxylate??

apparently being sold as dopaminergic nootropic stimulant: It is the piperazine analog of desoxypipradol (2-DPMP, 2-diphenylmethyl-piperidine aka Ivory wave) with the piperidine replaced by a piperazine.

It was patented in a old Dutch patent as a CNS stimulant. Can't pull that one out but I am sure I've seen it some years ago (would appreciate a link if anybody came across, thx).
Apparently, it is more potent than 2-DPMP as a NDRI and a cocnaine/MPH-like susbtrate but not AMPH-like inhibitor. Now, 2-DPMP is already one of the most potent NDRI iirc (active starting at 5mg-ish!). That means this compound may be active at micrograms range?!.. One advantage tho it may have shorter half-life than the insane 24h+ of 2-DPMP since the piperazine will decrease the logP by at least 2 log units relaltive to the parent piperidine! making it more polar and more easily excreted. Probably similar DOA to benzylpiperazine BZP (5-7 hours). Lower logP will also make it faster acting compared to highly lipophilic 2-DPMP that tend to accumate in fat and release slowy giving it slower absorption rate, longer half-life and irregular bioavailability. Plus the extra 4-nitrogen may make a nice metabolic handle for conjugation unlike with 2-DPMP or morphodrol (4-oxo-desoxypipradol, morpholine replacing the piperidine)... but I dont know until I try it!
Now, you might wonder why would that one be different from morphodrol which was a bust, had a pretty bad review at least with the nootropics crowd iirc? I don't know. But at least with the DAT transporter it seems a H-bond donor (not acceptor! like with morphodrol Oxygen) increases binding (relative to no H-bond group like in 2-DPMP) and a donor decrease it (like in morphodrol), but I am sure iirc the dutch patent describe it as potent CNS stimulant.. anyway, that is an interesting compound. Now, in that old dutch patent, they make it via pto2 reduction of 2-diphenylmethylpyrazine which itself was made from diphenylacetonitrile and 2-chloropyrazine. Pretty expensive route. Isnt other ways to make it? maybe in 2-step/1-pot from cheap diphenylacetonitrile and piperazine-2-carboxylate??