Soulfake
Bluelighter
- Joined
- Aug 12, 2010
- Messages
- 160
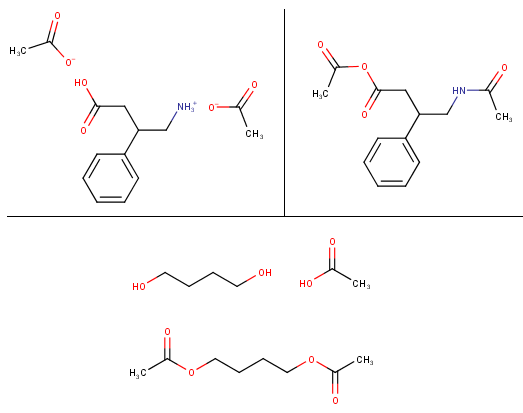
I have a hard time understanding the chemistry behind the different salts of drugs/substances (especially amino acids) and how exactly they are bound to the molecules and why/how they bahave in several ways. For example: If I want to make an free base e.g. from phenibut HCl I can add sodium bicarbonate or other bases (which ones could be used that are relatively safe, easy available and don't produce sodium chloride? For daily consumption table salt isn't the nicest impurity in drugs) but what about making a salt from e.g. caffeine or phenibut? As far as I read caffeine citrate is just made by mixing citric acid with caffeine in water (one recipe said that sodium citrate is added, too. It's an acid buffer but I also don't really understand how they work, I know they keep solutions at a certain PH range but I don't now how they behave in such salt-base reactions, when they should be added etc.), wouldn't then the caffeine from energy drinks, cola or coffee be bound to the acids that are in those drinks? Like caffeine phosphate from the phosphoric acid in cola or caffeine bound to the caffeic acid in coffee (caffeine caffeate?). Would this work for phenibut "free base" / free amino acid, too? So if I make a solution of the phenibut, neutralize it with baking soda and then add citric acid, will it produce phenibut citrate?
Would this also work with vinegar essence (25% acetic acid)? If you make a caffeine solution and let it dry it will give caffeine monohydrate which is like a cob web of fluffy long needles, I added some of the vinegar essence to some water-free caffeine and it seemed to have produced some kind of salt/caffeine acetate as it is now a white powder instead of that fluffly needle stuff. What I don't really get is why e.g. morphine that is treated with vinegar essence is at least partially converted to the acetyl-ester while other substances like caffeine just give the acetate salt. Would the reaction of phenibut FAA with vinegar essence give the acetate and how different is this reaction from making an hypothetical phenibut-acetyl ester?
edit: sry I should have posted this to the Neuroscience and Pharmacology Discussion board. Wasn't there one about chemistry?
Would this also work with vinegar essence (25% acetic acid)? If you make a caffeine solution and let it dry it will give caffeine monohydrate which is like a cob web of fluffy long needles, I added some of the vinegar essence to some water-free caffeine and it seemed to have produced some kind of salt/caffeine acetate as it is now a white powder instead of that fluffly needle stuff. What I don't really get is why e.g. morphine that is treated with vinegar essence is at least partially converted to the acetyl-ester while other substances like caffeine just give the acetate salt. Would the reaction of phenibut FAA with vinegar essence give the acetate and how different is this reaction from making an hypothetical phenibut-acetyl ester?
edit: sry I should have posted this to the Neuroscience and Pharmacology Discussion board. Wasn't there one about chemistry?
Last edited: