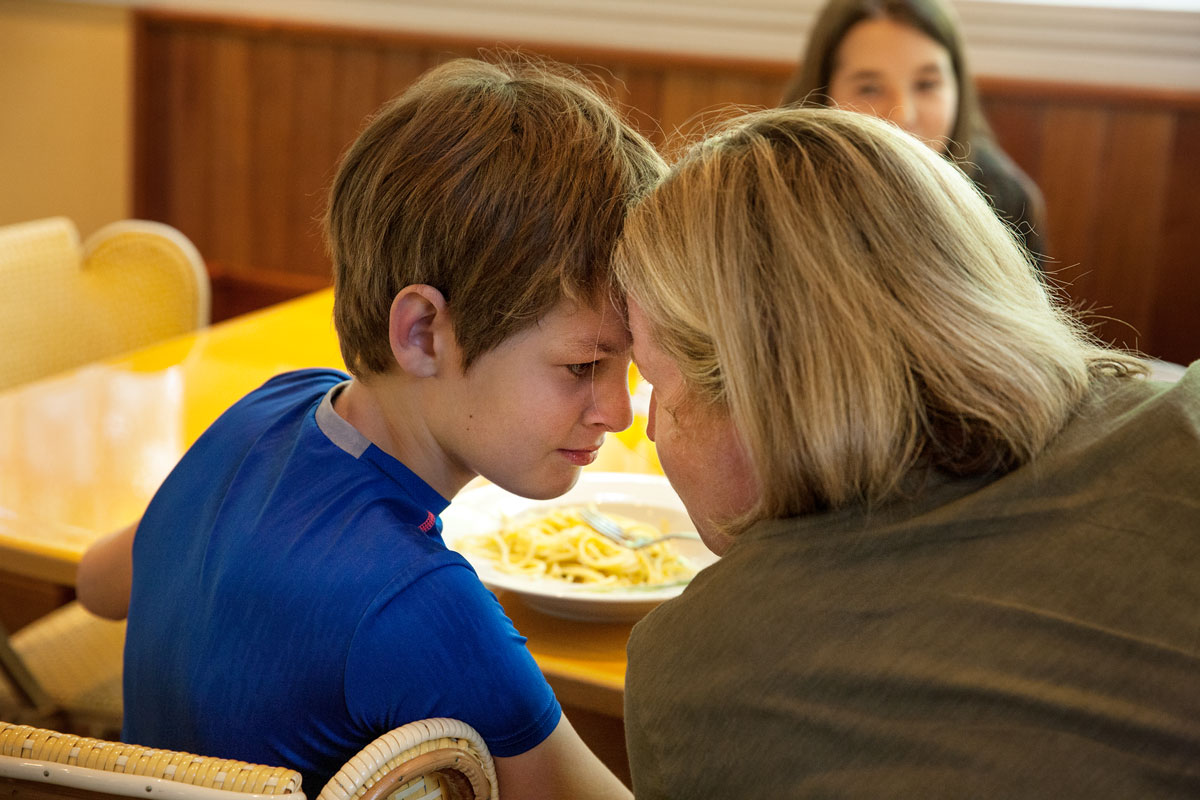Boy, interrupted
by Fred Vogelstein | WIRED
This is Sam. He’s my son. His epilepsy caused him to have up to 100 seizures a day. After seven years we were out of options. Our last hope: an untested, unproven treatment. The only problem? It was illegal.
The hospital pharmacist slid three bottles of pills across the counter, gave my wife a form to sign, and reminded her that this was not the corner drugstore. The pharmacy knew how many pills had been dispensed, he said; it would know how many had been consumed; and it would expect her to return the unused pills before she left the country. The pharmacist made it clear that he was not only in touch with our doctor but with the company supplying the medication. They would know if she broke the rules.
Evelyn said she understood and slipped the brown glass bottles into her purse. She and our 11-year-old son, Sam, were jet-lagged. They’d flown from San Francisco to London the previous day, December 19, 2012. Now, 30 hours later, it was just after 7 pm. They’d been at the Great Ormond Street Hospital for Children since midmorning. Sam had been through a brain-wave scan, a blood test, and a doctor examination. Some gel left in his hair from the brain scan was making him grumpy.
Evelyn was terrified. They’d come 5,350 miles to get these pills, medicine we hoped might finally quiet Sam’s unremitting seizures. He was to take a 50-milligram pill once a day for two days, increasing the dose to maybe three pills twice a day. Evelyn was to keep a log of his symptoms during their two-week stay. They would need to revisit the hospital two more times before they returned to San Francisco on January 3, 2013. That meant two more rounds of brain scans, blood tests, and doctors’ appointments.
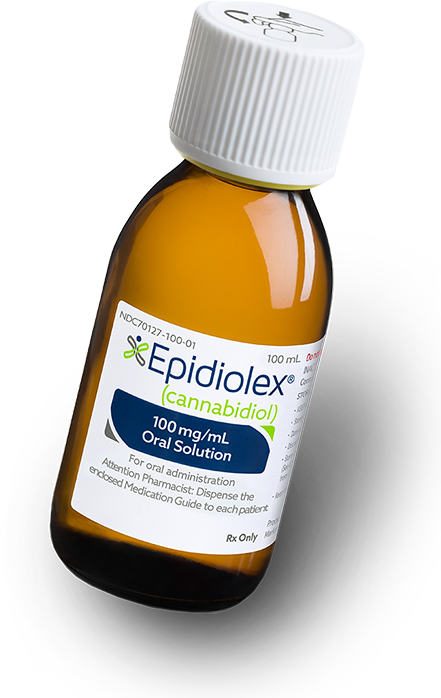
We were confident the medicine wouldn’t kill Sam or hurt him irreversibly, but the prospect still made us nervous. The pills contained a pharmaceutical derivative of cannabis. People have been smoking cannabis medicinally for thousands of years. Deaths are rare. But Sam would get a specific compound made in a lab. The compound, cannabidiol, known as CBD, is not an intoxicant. (Tetrahydrocannabinol, or THC, is the stuff in pot that makes you high.) Nevertheless, US drug laws made it nearly impossible to get CBD at this purity and concentration in the States.
It had taken four months of phone calls, emails, and meetings with doctors and pharmaceutical company executives on two continents to get permission to try this drug. Sam wasn’t joining an ongoing clinical trial. The company made the pills just for him. It believed CBD was safe based on animal studies. It also said it knew of about 100 adults who had tried pure CBD like this over the past 35 years. As a percentage of body weight, Sam’s dose would approach twice what anyone else on record had tried for epilepsy. Would it make him vomit or become dizzy, or give him a rash or cause some other unpleasant event? We didn’t know. We’d volunteered our son to be a lab rat.
Then there was a bigger question: Would the medicine work? No one knew. The reason Evelyn, Sam, and others in my family—including Sam’s twin sister, Beatrice, and Evelyn’s sister, Devorah—traveled to London during Sam’s winter vacation was that two dozen other treatments we’d tried had all failed. (I stayed behind in San Francisco, scrambling to meet an end-of-year book deadline.)
The one thing we were certain about: This was not going to be a bargain. We’d already spent tens of thousands of dollars on consultants to help Sam’s doctors set up the visit, and we were still at the starting line. The best-case scenario was that the medicine would work and eventually we’d be allowed to import it into the US. We secretly hoped that this would encourage the company to make the drug easily and cheaply available to others. We also knew this was quixotic. Our previous experience with medications suggested the whole venture would end in failure. This much we knew: Importing an experimental cannabis-based drug into the US would involve more than giving the company my address and FedEx account number.
If you’re the parent of a healthy kid, it’s hard to imagine yourself doing what we did. Who spends tens of thousands of dollars on anything that’s not a house, a car, or college tuition? Who lets their child be the first or even one of the first to try any medication? But Sam was not a healthy kid. He has had epilepsy since he was 4 and a half. We’d tried every possible drug—nearly two dozen medications—plus autoimmune therapy using intravenous immunoglobulin and a high-fat medical diet. (I wrote about our two-year diet experiment in The New York Times Magazine.) Little worked, and the treatments that showed some results didn’t work for very long or had worrisome side effects.
Sam doesn’t have grand mal seizures, the type most people imagine when they think of epilepsy: collapsing and twitching on the ground. Instead, he partially loses consciousness for five-to-20-second bursts. It’s a hard-to-treat variant of so-called absence epilepsy. The seizures themselves are more benign than grand mal, and they don’t leave him exhausted. But they are also much more frequent. When Sam’s seizures are uncontrolled he can have between 10 and 20 episodes an hour. That’s one every three to six minutes and sometimes more than 100 a day.
To me, watching Sam have a seizure looks like a movie that’s been paused and restarted. He stops and stares vacantly. His jaw slackens. And his head and torso lean forward slightly, bobbing rhythmically. Then it’s over, and he resumes life as if nothing happened. If he stopped walking, he’ll start again. If he was packing his backpack for school, he’ll continue. Though Sam says that he is sometimes aware when he has a seizure, typically his only clue is that when he comes to, everything around him has shifted slightly.
When they are frequent—which has been often—it’s hard for Sam to have a conversation, let alone learn anything in school. Sports? Not possible. As a little kid, Sam couldn’t even cry without being interrupted: He’d skin a knee, cry for 15 seconds, have a 15-second seizure, and then continue crying. Once, after watching a movie with me, he complained about the DVD being scratched. It wasn’t. It just seemed that way because he’d had so many seizures.
And while Sam got little help from the many antiepileptic medications that we tried, he endured plenty of side effects. One drug gave him hand tremors. Another made him violent. A third gave him hives. A fourth made him such a zombie that he drooled, while a fifth made him see bugs crawling out of holes in his skin. Twice his seizures were bad enough that we had to hospitalize him. He’d seen six neurologists at four hospitals in three states. I’ve seen him seize tens of thousands of times. You’d think I’d be used to it, but I find each one haunting—as if some outside force has taken over his body, leaving me, the person who is supposed to protect him, powerless.
By 2012, when Sam was 11, the only thing that was keeping his seizures controlled enough for him to attend school was massive doses of corticosteroids. If you or anyone close to you has had cancer, bad asthma, or any kind of major inflammation, you know about these drugs, which are synthetic versions of the body’s own anti-inflammatory compounds. Taken for a week or two, they can be lifesavers. But taken for extended periods, they wreak havoc on the body.
By the time he reached London, Sam had been on a big dose of the corticosteroid prednisone off and on for a year. It made him gain 30 pounds. It made his face look like it had been pumped full of air—a side effect known as “moon face.” And it weakened his immune system. He was starting to get head and chest colds every month. Were he to stay on these drugs at these doses longer-term, he would face stunted growth, diabetes, cataracts, and high blood pressure—all before he was old enough to vote.
So the trip to the UK felt like a last resort: If these pills got his seizures under control, he’d have as good a chance as any healthy kid to grow up to be a happy, successful adult. If they didn’t, well, we were out of options. He might grow out of his seizures, but there were no other medications or treatments that our doctors knew to try. It seemed hard to imagine him ever living on his own.
Sam’s situation is hardly unique. About 1 percent of the US population has epilepsy, and about a third of that 1 percent has epilepsy that can’t be curbed with medication. That’s nearly 3 million Americans with epilepsy and 1 million Americans with uncontrolled seizures. Epilepsy is more prevalent than Parkinson’s or multiple sclerosis. More than a dozen antiseizure drugs have come to market in the past 25 years. They’ve reduced the side effects associated with antiepileptics, but the new drugs haven’t proven much more effective at reducing seizures. The number of hard-to-treat cases of epilepsy like Sam’s hasn’t changed meaningfully in decades.
There are dozens of seizure disorders. Some cause patients to collapse like marionettes whose strings have been cut. Others cause a single limb to twitch. Big seizures can cause brain damage. And tens of thousands of people die every year from status epilepticus, a seizure that goes on for more than five minutes and typically requires a trip to the emergency room.
Think of a seizure as an overtaxed electrical grid. The human body is full of electricity that allows brain cells, nerves, and muscles to communicate in an orderly, controlled fashion. A seizure happens when this electricity spikes uncontrollably. As a result, parts of the brain’s circuitry temporarily shut down. You’d think medical science would be able to tell you why this happens and what to do about it, but with a few exceptions it can’t. Modern medicine can reattach fingers, replace a faulty heart, liver, or kidney, and regrow skin in a petri dish, but the brain’s abnormalities remain mostly mysterious and largely invisible.
Indeed, most epilepsy cases are like Sam’s, idiopathic, a fancy way of saying “no known cause.” A typical prognosis: If we can control the seizures with the first three meds, he’ll probably never have another one. If we can’t, the future is less certain. Beatrice developed absence epilepsy when she was older, in 2010. The first drug made the seizures disappear. She took it for two years. We have never seen another seizure.
There was nothing invisible or mysterious about Sam’s epilepsy in London, however. By the time he and Evelyn arrived, his seizure count was approaching its highest level ever. We had expected this. We’d reduced one of the drugs helping to control his condition five days before they left. If the drugs in London worked, we’d need convincing data to get permission to import them into the US. To get convincing data, we’d need to show a marked reduction in seizures.
It was not easy to watch. Two days before departure he had eight seizures. One day before departure he had 25. The day of departure he had 20, including 12 in the 88 minutes between 5:50 pm and 7:18 pm, immediately after the flight to London took off. By the end of the next day, when they picked up Sam’s pills at the Great Ormond Street Hospital pharmacy, his seizures had more than tripled to 68. Past experience told Evelyn that if the pills didn’t work fast, the following day would be a complete wipeout with more than 100 seizures.
The first time Evelyn and I talked about cannabis as a treatment for epilepsy was in early June 2011. The high-fat diet Sam had been on for two years had stopped working. There were no more conventional antiepileptic drugs to try. In our scramble to find solutions, Evelyn learned that a nurse-practitioner in one of our doctors’ offices was starting a cannabis collective—outside of work—to help some of the physician’s sickest kids. Other parents of epileptic kids we knew were joining. Besides having a medical degree, the nurse was an herbalist. She’d heard that cannabis—if made into oil-based tinctures, taken by the drop instead of smoked—could help people with intractable seizures. Evelyn liked the fact that the nurse sent her a 1981 paper from The Journal of Clinical Pharmacology on cannabinoids as potential antiepileptics. And she liked that the nurse assured her that the cannabis being used wouldn’t get anyone stoned. It would be high in CBD and low in THC.
Neither of us wanted to join the collective immediately. We had two other options for Sam we wanted to try first—corticosteroids and intravenous immunoglobulin. We also knew that if we were going to ditch Western medicine to treat Sam’s epilepsy, we’d have to do a lot more homework. Many people, often justifiably, hate drug companies. But one thing they are good at is making sure that every pill, drop, or spray of medicine they supply is exactly the same. Treating Sam’s epilepsy with cannabis would mean the reliability, consistency, and potency of his medicine was no longer assured.
My first reaction to the idea of trying cannabis to treat Sam was that it sounded crazy. I’d smoked plenty of weed in college and in my twenties. I knew the plant could have real medicinal effects; medical cannabis was legal to buy in California with proper documentation. But rightly or wrongly, the idea of controlling Sam’s seizures with cannabis—he was 10 at the time—alarmed me. I associated pot with partying, not treating my son’s serious illness. I hated having the two thoughts side by side.
But the desperate can’t afford to be doctrinaire. And by the time another year had passed, we were desperate. Intravenous immunoglobulin hadn’t worked. And it was becoming increasingly less safe to control Sam’s seizures with high doses of corticosteroids. In May 2012 we wrote a $600 check to join the cannabis collective.
We knew to expect uncertainty. Plants as medicine are by their nature variable in potency. The nurse was still trying to figure out which strains worked best and the optimal way to turn those strains into tinctures. And while some parents were reporting good results, no one was seizure-free.
But over the previous year we had also learned that treating epilepsy with cannabis wasn’t crazy at all. A small but growing body of research suggested that CBD might be a powerful anticonvulsant. Evelyn took particular note of a 2010 paper in Seizure, the medical journal of the British Epilepsy Association, that she found through a Google search. With charts and tables sprinkled over eight double-columned pages, the authors said that extensive tests on rodents in their labs, along with previously published data, “point to CBD being of potential therapeutic use (alone or as an adjunct) in the treatment of epilepsies.”
And then, remarkably, the first tincture we tried from the collective seemed to ratify those findings. For three days, Sam’s seizures went from what had been 10 to 20 an hour to about one every hour. The tincture was odd-looking—a bunch of cannabis leaves and stems in a brown mason jar marinating in oil. Using a syringe, we’d put a drop of the liquid on Sam’s tongue three times a day. It was supposed to be 20:1 CBD to THC.
But in July, coinciding with a new tincture, Sam’s seizures came roaring back. By the middle of the month he was having around 10 an hour. We tried increasing the dose. We tried tinctures bought at three medical cannabis dispensaries. They didn’t work either.
By mid-August we were thinking about putting Sam back on steroids. That was when the collective received test results for the latest batch of tinctures. They’d been advertised as having a 20:1 ratio of CBD to THC, but it turned out there was little CBD or THC in any of them. We also tested one of the other tinctures we’d bought from a supposedly reputable supplier. We’d been told it was 10:1 CBD to THC. It was really 3:1. The tincture that seemed to work for Sam in June hadn’t been tested, so we had no idea how to assess the temporary drop in seizures.
The experience with the unscientific methods of the collective and with the false labeling of products in dispensaries was infuriating and demoralizing. We knew the collective was still finding its way when we joined. And we knew that buying tinctures at dispensaries wasn’t like going to Walgreens. But somehow we convinced ourselves that the collective had mastered the basics—that you don’t tell parents a medicine is a certain potency unless you’ve had it tested. We really only had ourselves to blame, though. We didn’t have the tinctures tested either.
One parent we met through the collective decided to try to make a high-CBD tincture in her garage. Catherine Jacobson, whose son, Ben, also has epilepsy, has a PhD in neuroscience. She developed a method that took three days plus another five days of testing to produce a three-week supply.
It was anything but simple. She started by heating the cannabis for 30 minutes in her oven at 350 degrees to activate the THC and CBD. Then she put it in a plastic bag, crushed it, and dumped it into a beaker filled with ethanol. She let the mixture sit overnight on a stir plate, lab equipment which agitated the mixture, pulling the compounds out of the cannabis and into the ethanol. Then she strained it and put the ethanol on the stir plate for another eight hours until most of the liquid had evaporated. On the third day, she ran the mixture through a carbon column, using a vacuum pump. The column, which looks like a glass cylinder with carbon beads over a small opening at the bottom, separated CBD from the THC based on molecular weight. At the end of the process she’d have ten 10-milliliter test tubes. After testing, two or three would have a high-enough CBD-to-THC ratio to be usable. She’d concentrate those further to make medicine. Jacobson’s setup could only handle about a quarter pound of cannabis at a time.
That meant that if she started on a Friday night and spent all day Saturday, another half-day on Sunday, and waited another five days for test results, she’d have a 10-day supply of CBD the following weekend. The cost: about $750 for the cannabis and another $200 for ethanol. Two labs tested it at more than 100:1 CBD to THC. Ben and Sam seemed to respond to it. But she was only able to give us five days’ worth because it had been so labor-intensive to make.
Something else was happening throughout the spring and summer of 2012 that I only found out about much later. Evelyn had started wondering how to contact the head of a drug company in the UK. She’d been thinking a lot about that article in Seizure—the one that documented how pure CBD slowed seizures in rodents. But it wasn’t just the encouraging results that caught her eye. It was the authors, all researchers at the Schools of Pharmacy and Psychology at the University of Reading, one of the UK’s top research institutions. She noted that they had thanked GW Pharmaceuticals, a British company she’d never heard of, for funding the study.
GW, we soon learned, manufactured pharmaceutical-grade extracts of both THC and CBD. Its main business came from a drug called Sativex, which contains a mix of the two compounds in a mouth spray for sufferers of cancer pain or multiple sclerosis. But it also seemed to have supplied pure CBD to the authors of the Seizure study.
For Evelyn this was revelatory. CBD was the only thing left that might help control Sam’s seizures. And over in the UK there was a drug company making the stuff by the pound. The next move was obvious: Find out who ran GW—she quickly determined that his name was Geoffrey Guy—and figure out how to contact him.
She emailed the GW general mailbox and called the main phone number and left a message. No response. And then on August 17, 2012, we had a heated conversation with my dad. We were visiting him at his house in Wyoming, and he sat us down wanting to know what the next steps were with Sam. He was worried that the constant struggle was crushing our family. He was also worried that we’d given up, that in our desperation we’d become like acrophobic climbers, terrified to change our position on a cliff despite only being 5 feet off the ground. That was painful to hear, but it also gave Evelyn an idea. She came to breakfast the next day and said,
“If you really want to be helpful, get us in touch with Geoffrey Guy.”
And that’s what he did. His firm, Warburg Pincus, had been doing business in London for 25 years. On August 20 he emailed some associates, detailing Sam’s situation. Eleven days later Geoffrey Guy wrote to Evelyn asking how he could help. Later that day he told Evelyn on the phone that figuring out a way for Sam to try GW’s CBD was eminently possible, and that he would do what he could to be helpful.
What we didn’t know at the time was that Guy and his team had already been wondering about human trials with CBD for epilepsy. And it turned out that the kind of one-patient experiment we were suggesting wasn’t unheard-of in the UK. Doctors there can get promising medications for their patients from the manufacturer to be used under their direct responsibility. It’s known as administering on a named-patient basis. No regulatory approval is required as it is in the US. Guy said he’d done it with more than a thousand patients in his career.
“I have been looking for a number of years at using CBD in just such a situation,” Guy says.
“You are a parent of a child who had a specific need. All other medicines had failed to help. We had a medicine that might help. Why on earth would that not be a good and wholesome thing to do?”
The catch was that GW would only consider helping us get CBD for Sam if we did it completely aboveboard. We couldn’t try the drug in the US. We’d have to go to the UK. We’d need our US doctor’s permission. We’d have to find an epilepsy doctor in London to take our case and to agree to supervise the treatment and various tests.
And if the medication worked, we’d need to navigate a labyrinthine approval process to legally import the drugs into the US. The research ethics committee at our doctor’s employer, UC San Francisco, would have to approve our plans to administer the medication at the hospital. Would a public institution like UCSF, dependent on federal research grants, agree to oversee treatment with a quasi-legal drug? The US Food and Drug Administration would need to sign off on what we were doing. The FDA has a process for individuals to get approval to try unapproved drugs on a so-called compassionate-use basis. We’d heard the applications were typically hundreds of pages long.
And then we’d need clearance from the US Drug Enforcement Administration. The head of the DEA at the time, Michele Leonhart, took a hard line on cannabis, which to this day remains listed as a Schedule I drug, supposedly as dangerous and addictive as heroin. Despite legalization efforts in some states, it’s the federal government that controls the borders, and to get any illegal drug across the border you must get approval from the DEA.
The magnitude of the undertaking was daunting, not to mention the cost. Just traveling to London, staying for two weeks, and paying doctors’ bills out of pocket would run into the thousands. We’d have to hire consultants to draft our applications to the FDA and DEA. Our doctor hadn’t done anything like this before. The only way she was going to be able to get behind it on our behalf was if we handled all the paperwork for her.
Back then, UK regulators said they would never approve it. But by the middle of the decade, the British political landscape had shifted significantly. The courts were clogged with the cases of multiple sclerosis and cancer patients who’d been arrested for using cannabis to combat things like muscle spasticity and the nausea from chemotherapy. Politicians and activists were calling for partial legalization.
And so, in July 1997, Guy found himself at a joint conference of the Royal Pharmaceutical Society and the Multiple Sclerosis Society. Onstage were top UK doctors and regulators wondering aloud what it would take for a company to make a cannabis pharmaceutical. Guy raised his hand and explained how he thought it could be done. A year later, in June 1998, Guy and cofounder Brian Whittle got permission to start GW.
“It was like a wormhole opened up and we’d spent the previous 10 years studying wormholes,” Guy says.
By 2012, GW was one of only a handful of firms in the world doing legal, drug-company-quality research on cannabis. It owned enormous hothouses containing thousands of cannabis plants in legal but undisclosed locations southeast of London. It had modern labs converting the plants into medicinal extracts and a factory that could turn them into sprays, tinctures, and pills. It had 177 employees and $51 million in revenue. And it was manufacturing its first drug, Sativex, already approved for sale in the UK, Canada, and 22 other countries to treat MS.
Back in the UCSF conference room, we finalized our plans: Evelyn would take Sam to London, where he would try pure CBD pills made especially for him. He wouldn’t be the first person to try pure, pharmaceutical CBD for epilepsy. Four small studies between 1978 and 1990 had tried it on a total of about 40 people. Surely others had tried homemade concoctions. But he’d certainly be the first kid, and arguably the first person in more than 20 years, to try CBD of this purity for epilepsy. We hoped it would work for Sam and that many other patients like him would follow.e met Geoffrey Guy face-to-face in a conference room off the eighth-floor neurology waiting room at UCSF. We were dressed like we lived in California. Guy was dressed like an early-20th-century English banker. He wore a double-breasted suit, a white-collared blue shirt with French cuffs, and a yellow tie with blue polka dots. Evelyn and I had been exchanging emails with him since the end of August 2012. Now, in early December, we were sitting down to discuss some last-minute details of our London trip. The meeting was also an opportunity for Guy to talk with Sam’s new doctor, Roberta Cilio. Sam’s longtime neurologist had had to take an emergency leave of absence the week before. Cilio, an eminent Italian physician who had only joined the staff of UCSF the previous September, was jumping into the middle of an unfamiliar case. We were meeting her for the first time too.
We knew little about Guy at that point other than the essentials: He was a longtime biotech entrepreneur, and he had an experimental compound that, turned into a drug, might help Sam.
We learned later that he’d started three notable drug companies and brought more than a dozen medicines to market. He knew more about cannabis than almost any executive in the world. And in more than 30 years as a biotech CEO he had built a reputation as a maverick—someone attracted to the thorny, controversial pharmacological issues that most executives try to avoid. Guy had been thinking about starting a company to make medicines from cannabis since the early 1990s.
Settling into London, Evelyn was almost afraid to believe how well the treatment seemed to be working. After having 68 seizures on Thursday, the day Sam and Evelyn spent at the Great Ormond Street Hospital, Sam had 10 on Friday and five on Saturday, 10 on Sunday and six on Monday. And as she increased the dose of CBD from 50 mg a day to 250 mg a day, his seizure count continued to fall. They didn’t see any side effects.
Sam’s seizures decreased so quickly—in less than 24 hours—that two days after he took his first pill he was zip-lining 30 feet in the air above a carnival spread out over half a mile in Hyde Park. He was harnessed, so he couldn’t fall. And because he has a weakness for the scariest ride in any amusement park, Evelyn couldn’t say no.
At first we said nothing to family or friends. We worried that, like so many promising treatments we’d tried, the effect would be only temporary. But by December 28, 2012, eight days after Sam’s first pill, it was obvious that we were witnessing something fantastic. “Best day yet,” Evelyn wrote to friends and relatives.
“Today Sam had a total of three seizures—short, seconds-long. From our starting point of SIXTY-EIGHT seizures. I would say we are doing very well. And we can go higher on the dose if we like. Along with being nearly seizure-free, Sam is more mature, more relaxed, and funnier. No idea if that is a physiological effect or just a result of not having his train of thought interrupted all the time, but who cares … I love to see all that come out.”
Our euphoria lasted only two weeks. The trial was ending, and Guy wasn’t going to let us take any CBD back to the US. On January 2, 2013, he emailed Evelyn telling her that he’d be sending one of his executives by the hotel to pick up the unused pills.
We knew to expect this, but it was still excruciating. Two weeks into enjoying the best seizure control of Sam’s life, we were being asked to give back the medicine that got him there. We had cobbled together a plan to manage Sam’s seizures during the time it would take us to obtain permission and cooperation from UCSF, the FDA, and the DEA. We hoped that would happen in less than six months, as we’d been told. But no one knew for sure. When you’ve found the first medicine in seven years that controls your child’s debilitating illness, not having it for an hour feels too long.
What we used to control Sam’s seizures during that time seems irresponsible in retrospect. A few weeks before we left for London we’d found an outfit in Colorado that claimed to make CBD pills from hemp. They seemed to help Sam a bit, and the company was willing to send the pills through the mail. Testing showed a CBD-to-THC ratio of 18:1. But we didn’t have any idea where they were getting their raw material. We didn’t know whether their manufacturing process was clean. A month’s supply cost more than $1,000. At the time it seemed better than putting Sam back on steroids.
The agents from the DEA appeared without an appointment at Cilio’s UCSF office door on March 1, 2013. They showed her their badges, asked permission to question her, and made it clear that this was not going to be a friendly conversation.
“They asked a lot of personal questions: Where was I from? Had I ever used (illegal) drugs?” she says, adding that it made her feel like she was part of a TV crime drama.
The questioning, which went on for two hours, got particularly tense when the agents asked Cilio how she planned to dispense the special drug. “I said that I would keep it here in my office and then put it in my purse and walk across the street to the clinic to see my patient. And they said, ‘You have no idea what you are talking about. This is a Schedule I drug. It’s like heroin. You can’t cross the street with it in your bag. You have to keep it in your office, and you have to give it to the patient in your office.’”
As part of the special license application process, she’d been briefed by UCSF security and demonstrated to the agents that she understood how the locks and alarms worked in her office suite and building. They weren’t satisfied with this either. They took pictures of the furnishings in her office, including the cabinet she told them she was going to store the medicine in. They told her she would need a safe.
I was both elated and panicked when I heard about the agents’ interview. Setting up a site visit by agents is notoriously slow. Our application had been live for only about eight weeks. But I worried the DEA’s demand for a safe would suck our application into a bureaucratic quagmire. I had initially thought the agency would be satisfied with a cheap jewelry safe I could buy at the hardware store. I was wrong.
According to government regulations, the safe needed to be certified for
“30 man-minutes against surreptitious entry; 10 man-minutes against forced entry; 20 man-hours against lock manipulation; and 20 man-hours against radiological techniques.” What this means in English is a 3-foot-square steel box that weighs 965 pounds and looks like something the Road Runner used to drop on Wile E. Coyote. But it never occurred to me that you could buy one. Would UCSF actually pay for something like this? Even if it would, I envisioned months of paperwork just to get the university’s approval.
It turns out that used safes are not hard to find. Cilio said that if I bought one, she’d gladly put it in her office. UCSF officials said it was OK as long as the safe didn’t violate building load limits. And within a day I was the proud owner of a used blue Meilink TL-15 plate safe with a group 1R lock. And by week’s end I’d had it delivered to Cilio’s office. The cost for unraveling this bureaucratic knot: $2,100.
The DEA approved our application on March 19. Between getting import permits, clearing customs, and Cilio’s unrelated attendance at an overseas conference, another six weeks passed. Sam took his first CBD pill in the US on May 4, three weeks before his 12th birthday.
The total bill for getting GW’s CBD into the US was roughly $120,000, not including travel. Two consulting firms—one an expert in the workings of the FDA, the other an expert in the DEA—generated most of those expenses. It’s an enormous amount of money to pay for outside help, more than double what we’d thought it would cost.
But it’s hard to imagine how we could have done it without them. Cilio had dozens of other patients to attend to besides Sam. And because she was new to the US, she had no idea how complicated and emotionally charged anything associated with cannabis can be here. The consultants showed her how to fill out the mountain of paperwork involved in applying to the FDA and the DEA. And they worked their contacts inside the agencies to make sure our application kept moving. The DEA agents, despite being antagonistic with Cilio and demanding that we get a safe to store Sam’s drugs, also moved our application along quickly when we speedily met their demands. The day we had the safe delivered to Cilio’s office, an agent visited to ensure it met DEA requirements. And he immediately advanced our application to the next step.
We wouldn’t have even known that consultants did work like this had Steve Willard, a Washington, DC, drug company entrepreneur, not introduced us to them. Sam now says he’s his best adult friend, even though he was my dad’s friend first.
Usually, getting access to experimental drugs that are potentially lifesaving doesn’t work this way. With terminal cancer patients, for example, oncologists know what new drugs are in development and have a mechanism already established to work with a company and quickly get FDA approvals. Yet GW was supplying drugs that were illegal in the US. No US hospital would take on a project like this.
But it appears our enormous bill for helping Sam has also jump-started the development of what doctors tell us could be one of the most exciting new drugs to treat epilepsy in a generation. Within a month of our return from London in early 2013, Guy and GW started talking to epileptologists at four other US hospitals about doing studies with their sickest kids. And on January 26 in New York City, 15 doctors, researchers inside and outside the US government, and GW officials sat in a conference room at NYU and began mapping out a strategy.
Those initial investigations—five hospitals, 25 kids apiece—proved so encouraging that GW last year expanded them to what it expects will be 1,400 patients at more than 50 hospitals in the US and the UK by year’s end. The drug now has a name—Epidiolex—though for a day or two Guy talked about naming it after Sam. It has a fast-track designation from the FDA, meaning that it could be available at Walgreens inside of three years.
Epidiolex is not a miracle cure. The most recent data, out in April, shows that of 137 kids who tried it for 12 weeks, it helped about half, reducing their seizures by at least 50 percent, with 9 percent becoming seizure-free. This is a better response rate than it sounds. All of the patients in the trials are those like Sam who had already run out of conventional options. But it is also a reminder that CBD, Epidiolex, or any seizure drug doesn’t help everyone.
Today CBD’s potential for treating epilepsy has become an important story in medicine. In August 2013 Sanjay Gupta, CNN’s chief medical correspondent, reported on a cannabis strain that had all but cured Charlotte Figi, a 5-year-old girl with Dravet syndrome, one of the worst kinds of epilepsy. Figi was in a wheelchair, on a feeding tube, with a do-not-resuscitate order before her parents started experimenting with high-CBD cannabis in 2012. The oil, supplied by a group of evangelical Christian brothers in Colorado Springs named the Stanleys, helped her almost immediately. Figi quickly went from 300 grand mal seizures a week—an average of 40 a day—to about four a month.
That cannabis documentary on CNN, a second in 2014, a third in April this year, and a week’s worth of editorials in The New York Times in 2014 have ignited a national conversation not just about CBD for epilepsy but about whether it is time to legalize cannabis entirely. Twenty-three states have legalized medical cannabis, 18 states have decriminalized recreational cannabis too, and four states have made recreational use completely legal. Expect at least five more states, including California, to put complete legalization to a vote in 2016. And bills in Congress to change the laws on a federal level, which at a minimum would make it easier for researchers to study cannabis in the lab, are getting traction for the first time.
Edward Maa, a neurologist at the University of Colorado, Denver, is doing the first study of the Stanley brothers’ strain, now called Charlotte’s Web, to get data on its effectiveness. He has 14 Dravet patients so far. The Stanleys now ship Charlotte’s Web across state lines, because the cannabis has so little THC that it is considered hemp. The operation has 3,508 customers, about a third of them kids with epilepsy.
Little of this was happening four years ago, when Evelyn and I first started using cannabis and epilepsy in the same sentence, and watching Sam’s life unfold alongside it has been profound. Sam isn’t seizure-free, but he’s close, like he was in London. He has between zero and five seizures a day, and he’s been off all other antiepileptic medications for almost two years. GW makes Epidiolex only as a liquid now. Sam takes 3.5 ml at breakfast and dinner. Evelyn and I are still thinking about how we can eliminate the last of the seizures. Sam is, perversely, more frustrated by these episodes than when he was seizing every few minutes. Back then he was in a fog. Now, because he is so close to being seizure-free, he feels each disruption more keenly. He increasingly understands that if we can’t get the remaining few to go away, he won’t be able to drive or ride a bike.
But for the first time in a decade—since he was in preschool—he is living like a normal boy. He takes a bus and a train home from school in San Francisco every day. He’s studying to be a bar mitzvah next year. He plays Halo at his friend Brian’s house on Friday afternoons. Before school let out for the summer, he was doing more sports than he actually had time for. He fences three times a week. He was on his school’s indoor soccer team. And he runs a nine-minute mile. Sure, he’s about 5:15 off world-record pace. But he could barely run 100 yards without seizing three years ago. Last summer we went fly-fishing and rock climbing with ropes. He makes up songs in the morning before school.
And he is turning out to be a smart, thoughtful kid. Lately on car rides he asks me questions like this:
“Why does everything have to cost money? Why can’t it all be free? Money is just paper. What do we need it for?” Or
“Why are we here? Where did we come from?” He is arguably late asking such questions, but that doesn’t bother me. For most of the past decade I was worried Sam would never be well enough to even formulate thoughts like this. And he was very clear about the fact that he wanted me to write this story.
“People need to know what this looks like,” he told me when we discussed it. He recalled one of his earliest memories of having seizures in kindergarten: “Do you know what it’s like to be line leader, have a seizure, and wake up with everyone yelling at you?”
Listening to him talk like this or hearing Evelyn recall her conversations with him is bittersweet. Most of us spend our childhood blissfully thinking that our parents can solve most of the big problems we face. Sam had to find out way too early that sometimes that’s just not true. I hate that he had to learn that lesson so soon. I hope surviving it will give him the inner strength to better handle life’s other beanballs.
All of it makes me recall a conversation I had in 2009 with Doug Nordli, an eminent Chicago epileptologist. He made a point of saying that, as hard as we might find it sometimes, the one thing we should never do is become hopeless about Sam’s seizures. This wasn’t just a pep talk. He said that he’d seen kids like Sam rebound with astonishing speed once their seizures were brought under control. I wanted to believe him, but back then I just couldn’t. Now I see proof every day of how wrong I was.
My son's epilepsy caused him to have up to 100 seizures a day. Our last hope: an untested, unproven treatment.
www.wired.com