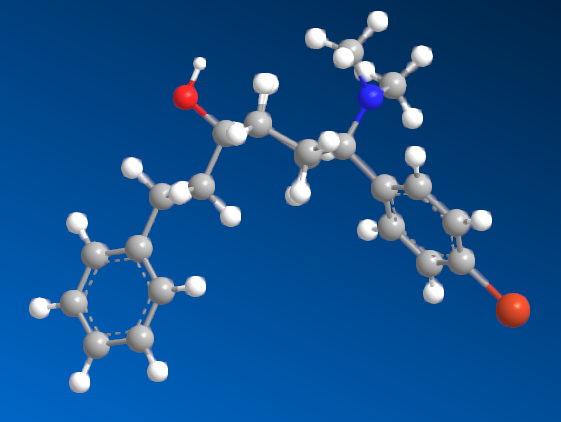
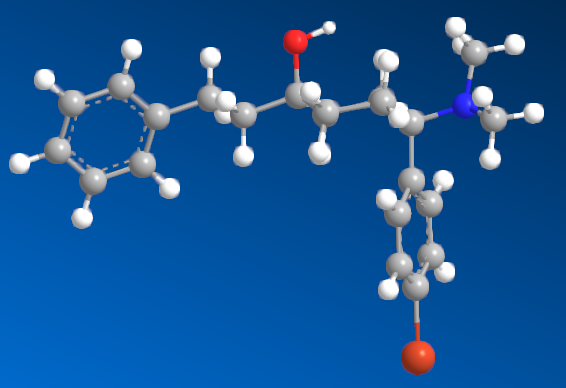
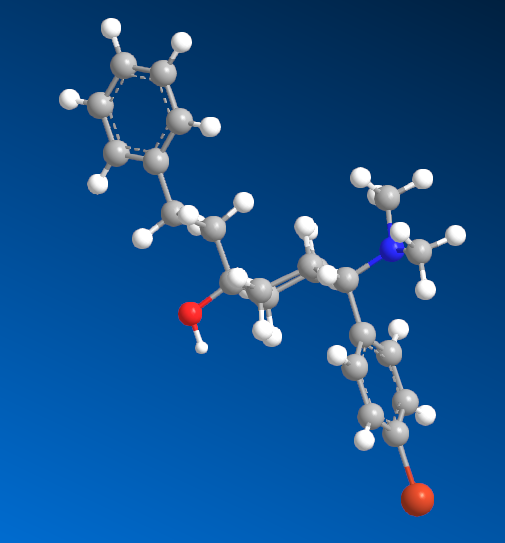
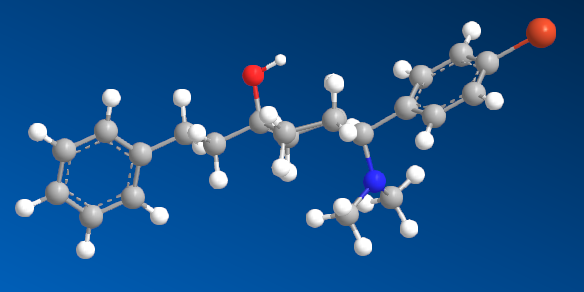
I'm sure that anyone with a keen interest will own a copy of 'Opiates' by Lenz et al. Page 441, section D. 'Benzylamine-Based Compounds'. 5-(dimethylamino)-4,4-dimethyl-1,5-diphenylpentan-1-one is listed as equipotent with codeine. What is of vastly more interest is the fact that it's structure is midway between BDPC (and I assume C-8813) and the 3-Amino-3-phenylpropionamide class. Ciramadol may be considered the lead compound and so my first question is, do people think that this scaffold is chiral? It's important to grasp how important the phenol (or bioisostere of same) is. I don't believe it's possible to produce an antagonist without a phenol (for example). One epimer of Alvimopan, meptazinol & picenadol all display agonism (sometimes silent agonism) while another is an antagonist. What they all display is a dearth of useful data when used in a training set.
The dimethyl moiety (or biostere) should be familiar from some very old opioids (like 3,3-dimethylpethidine, 3,3-dimethylprodine dimethylaminopivalophenone, 3,3-dimethylpropiram) but as you can see from the image, it is a key moiety but like the alkene (allylprodine, cinnamyloxycodone), no software deals with it. What IS important is that wherever a chiral methyl (typically) moiety is present, in every case pethidine to fentanyl, the 3,3-dimethyl is as potent (per mol) as the if having a slightly higher MW (due to extra -CH2). Anyone who has studied the Eunoia disc will be aware that derivatives of propiram, phenapromide & diampromide all perfectly overlay fentanyl. There is no bulk preventing the dimethyl (or indeed a -CH3 & -OH at the 3 position were found to be active) from binding... it just means 2 isomers, not 4.
So I post this just to see if others can further evolve a QSAR for the class. The benzyl amine is the BIG difference. As people may be aware, Dr. Lednicer first made pethidine-like compounds with a 4-piperidone aromatic with the benzyl dimethylamine and was rather surprised. I does however seem like others have stumbled upon the same things. Compound 82 may benefit from a p-NO2 on the benzophenone moiety and I'm pretty sure a dimethyl on the phenylethyl propanamide class (Itself brushing the benzamide opioids like U-47000). We know that a 2-(2-thienyl)ethyl moiety (see C 8813) increases potency by almost exactly the same % as thiofentanil is from fentanyl. That being the case, Jansen's exhaustive experiments on that ethylaryl moiety uncovered the fact that an N-2-(1,3-thiazole)ethyl moiety produces a compound some x4 of it's parents but also a duration x4 of it's parent.
Sorry to ramble on but if there are compounds you think should be added, please let me know - it's all going into a training set (and everyone will have access).
https://imgur.com/a/XnqoVho
PS I vaguely remember a weak (400mg = 8mg codeine) opioid which was an N,N-dimethyl-1-phenyl-1-pyridinyl)methanamine. I forget where the N went and the search engine sucks. Can anyone identify it as it neatly links propiram to the others.
The dimethyl moiety (or biostere) should be familiar from some very old opioids (like 3,3-dimethylpethidine, 3,3-dimethylprodine dimethylaminopivalophenone, 3,3-dimethylpropiram) but as you can see from the image, it is a key moiety but like the alkene (allylprodine, cinnamyloxycodone), no software deals with it. What IS important is that wherever a chiral methyl (typically) moiety is present, in every case pethidine to fentanyl, the 3,3-dimethyl is as potent (per mol) as the if having a slightly higher MW (due to extra -CH2). Anyone who has studied the Eunoia disc will be aware that derivatives of propiram, phenapromide & diampromide all perfectly overlay fentanyl. There is no bulk preventing the dimethyl (or indeed a -CH3 & -OH at the 3 position were found to be active) from binding... it just means 2 isomers, not 4.
So I post this just to see if others can further evolve a QSAR for the class. The benzyl amine is the BIG difference. As people may be aware, Dr. Lednicer first made pethidine-like compounds with a 4-piperidone aromatic with the benzyl dimethylamine and was rather surprised. I does however seem like others have stumbled upon the same things. Compound 82 may benefit from a p-NO2 on the benzophenone moiety and I'm pretty sure a dimethyl on the phenylethyl propanamide class (Itself brushing the benzamide opioids like U-47000). We know that a 2-(2-thienyl)ethyl moiety (see C 8813) increases potency by almost exactly the same % as thiofentanil is from fentanyl. That being the case, Jansen's exhaustive experiments on that ethylaryl moiety uncovered the fact that an N-2-(1,3-thiazole)ethyl moiety produces a compound some x4 of it's parents but also a duration x4 of it's parent.
Sorry to ramble on but if there are compounds you think should be added, please let me know - it's all going into a training set (and everyone will have access).
https://imgur.com/a/XnqoVho
PS I vaguely remember a weak (400mg = 8mg codeine) opioid which was an N,N-dimethyl-1-phenyl-1-pyridinyl)methanamine. I forget where the N went and the search engine sucks. Can anyone identify it as it neatly links propiram to the others.