(zonk)
Bluelighter
- Joined
- May 24, 2008
- Messages
- 687
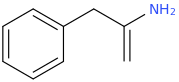
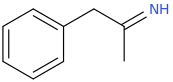
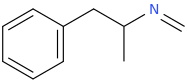
I was wondering if any research has been done or if anyone could speculate as to the activities of meth/amphetamine analogs where either an allyl bridge is used with or without an alpha substitution or if the bridge can be reversed to have the extra carbon closer to the alpha position in place of the standard propyl or i Also can't locate [anything] on 1-halophenethylamines which i thought was surprising. I was thinking alpha-chloro or fluoro