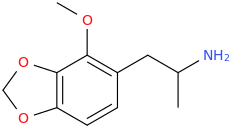
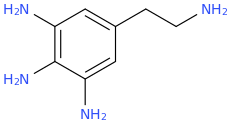
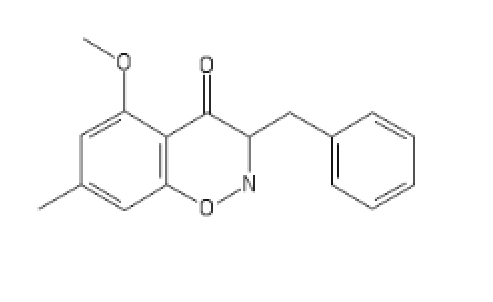
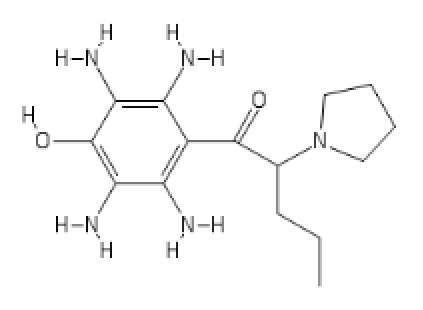
-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Dresden's Chemical Fluff Thread (Name-A-Molecule)
- Thread starter Dresden
- Start date
Limpet_Chicken
Bluelighter
- Joined
- Oct 13, 2005
- Messages
- 6,323
Well to be fair, group I metals aren't really the thing one can effectively sneak into somebody's brew and spike their drink with them.
Unless a mug half full of vac pump oil and me standing round a corner wearing my blast shield, goggles, and with both my fingers in my ears, grinning the sort of grin that's generally considered a viable reason to shoot someone first, and find out why you did it during an autopsy
I do have some good tea though, couple of green teas, loose and bagged, forget the brand as I don't care for green tea much, black with rose essential oil in it, lady grey, earl grey, a really nice delicate white tea that goes great with a handful of lemon balm, for some reason there's also PG tips, and a decaff version of that. I'd sooner swallow the mug full of NaK to be honest, no idea how it got there, probably that fucking psychobitch former housemate from several years back, the one who's a total mental borderline PD bitch, rapid cycling bipolar, a compulsive liar and klepto, the one that cried rape falsely against people she decided (not me) to use until they had no more to give, and eventually tried to run me through with a samurai sword whilst I was mostly naked, possibly entirely so, I can't quite remember, it was several years ago, thankfully)
Had to have been that waste of a set of spesh wetware, because I'm the only one that drinks tea at all here, and I wouldn't drink that filth any more than I would anything tea or coffee based with detectable milk contamination
No guarantees about the absence of pre-prepared tea with agar added, or served with a spoon cast out of galinstan. The look on someone's face after they try stirring in their sugar, and pull out just a little wee nub of rapidly liquefying metal..that one is always hilarious. Should have seen my grandmother's face when I got her with an agar-laced solidified tea made hours in advance. Now that was funny
Unless a mug half full of vac pump oil and me standing round a corner wearing my blast shield, goggles, and with both my fingers in my ears, grinning the sort of grin that's generally considered a viable reason to shoot someone first, and find out why you did it during an autopsy

I do have some good tea though, couple of green teas, loose and bagged, forget the brand as I don't care for green tea much, black with rose essential oil in it, lady grey, earl grey, a really nice delicate white tea that goes great with a handful of lemon balm, for some reason there's also PG tips, and a decaff version of that. I'd sooner swallow the mug full of NaK to be honest, no idea how it got there, probably that fucking psychobitch former housemate from several years back, the one who's a total mental borderline PD bitch, rapid cycling bipolar, a compulsive liar and klepto, the one that cried rape falsely against people she decided (not me) to use until they had no more to give, and eventually tried to run me through with a samurai sword whilst I was mostly naked, possibly entirely so, I can't quite remember, it was several years ago, thankfully)
Had to have been that waste of a set of spesh wetware, because I'm the only one that drinks tea at all here, and I wouldn't drink that filth any more than I would anything tea or coffee based with detectable milk contamination
No guarantees about the absence of pre-prepared tea with agar added, or served with a spoon cast out of galinstan. The look on someone's face after they try stirring in their sugar, and pull out just a little wee nub of rapidly liquefying metal..that one is always hilarious. Should have seen my grandmother's face when I got her with an agar-laced solidified tea made hours in advance. Now that was funny

Limpet_Chicken
Bluelighter
- Joined
- Oct 13, 2005
- Messages
- 6,323
Xmas trees are great fun to set on fire. The blaze just rockets upwards.
And instead of an angel on to, why not make it a tank of acetylene or MAPP gas plus a portable O2 cylinder, wrapped up in a mix of potassium chlorate, sugar, a bit of salt to slow the burn, and wax to bind it, plus say, finely powdered ammonium nitrate added in for good luck.
Guaranteed to make sure one's xmas goes with a bang
And instead of an angel on to, why not make it a tank of acetylene or MAPP gas plus a portable O2 cylinder, wrapped up in a mix of potassium chlorate, sugar, a bit of salt to slow the burn, and wax to bind it, plus say, finely powdered ammonium nitrate added in for good luck.
Guaranteed to make sure one's xmas goes with a bang

Limpet_Chicken
Bluelighter
- Joined
- Oct 13, 2005
- Messages
- 6,323
Bad idea. Aromatic amines are often toxic, unpleasant things.
Dresden
Bluelighter
- Joined
- Feb 2, 2010
- Messages
- 3,212
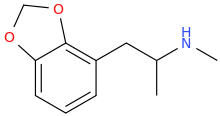
DAVE ABRAHAM
Look, I can draw one Markush structure for 80% of Dresden's doodles.
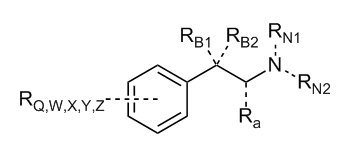
Maybe I can file a patent.
Phen Again, huh?
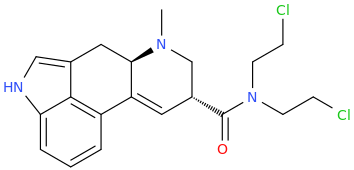
JAMES JERNIGAN
This will fit its receptor better.
Could we?
Should We?
Au, this thread is a veritable GOLD MINE.
A Star Is Born:
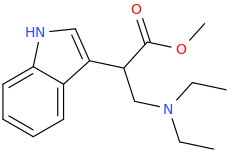
KRYPTA
THE CONTINUATION CONTINUES.
Last edited by a moderator:
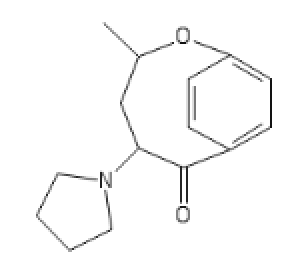
I'm surprised that 2-Br LSD (or LSD analogue) isn't an item of commerce. No activity whatsoever and a gentle reduction yields active in 78% yield (I guess you just leave it in there). It isn't an API but it means people can make the immediate precursor on a large scale for people to finish.
https://imgur.com/a/H3QZvQf
I just thought I would post a 'novel' opioid based on several Janssen patents. It turns out a carbamate ester is a bioisostere of an amide in this case (goodbye MoDA), that 3,3-disubstitution is fine and unless you are going to resolve the enantiomers, is more potent than 3-MF and if you DO resolve, it's only the lower MW of 3-MF that makes it marginally stronger. The thiazole proves to be as potent as the 2-furyl (i.e. twice as potent as the 2-thienyl) and unexpectedly has a duration some x4 longer. I can only presume that the liver enzyme(s) responsible for N-dealkylation have trouble with that aromatic (oxazoles also work but the LogP isn't as favourable). The o-F is just my little joke, 2-fluoroaniline just means 1 more thing to watch but it isn't like more resources appear to actually DO so.
In short, while NPP (N-phenylethyl-4-piperidone) is still the precursor-of-choice (and is watched), 3,3-dimethyl-1-[2-(1,3-thiazol-2-yl)ethyl]piperidin-4-one is an item of commerce. Good luck with the ethyl chloroformate!
It's purely given as an example of just how easy it is to avoid the MoDA and 'watched' precursors. That people aren't even bothering kind of hints that control isn't working AT ALL. That does anger me since fentanyl has become a disaster area with 1000s of people ending up dead from C cut with F. I suspect the authorities aren't really trying very hard.
-----
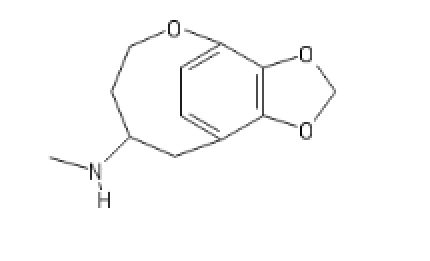
https://imgur.com/a/JYeLkLt
Mate in 3. I checked with the Home Office and as long as the precursor is intended as a precursor, not as a medicine, I don't even need a licence. OK, it's 3 steps (as opposed to 2 for fentanyl) but that 14-methoxy appears to produce a LOT of delta activity even in the absence of the ether bridge. I'm just pointing out that some REALLY potent stuff is within anyone's reach. The MoDA doesn't cover it! I'm not defending people making this stuff, I'm just appraising people of the fact that it's not exactly difficult to make the stuff and where there is ? to be made, people will.
I admit that the alkylation of the tertiary alcohol is a bit of a pain. Schmidhammer does at least go for an 'uneventful' route but it's and interesting problem. In this case, the absence of certain moieties does make the scaffold more robust (that bridge is a sod for breaking).
-----
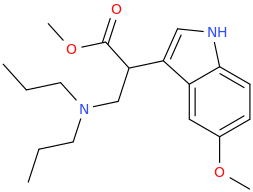
https://imgur.com/a/SKCnQFC
One for the training set. It overlays BDPC spectacularly well although Dr. Lednicer had never come across it. So will a m-OH or p-Br work? I'm going to say YES. Will replacing the acetophenone moiety with a different aromatic work. I'm going to say yes. Dimethylaminopivalophenone follows the classic mu training set but like BDPC, this compound shows that you can move that basic nitrogen. I vaguely remember another scaffold with a secondary amine that overlaid BDPC but it had to much sigma affinity. Still, it's all good for your training sets.
I just thought I would post a 'novel' opioid based on several Janssen patents. It turns out a carbamate ester is a bioisostere of an amide in this case (goodbye MoDA), that 3,3-disubstitution is fine and unless you are going to resolve the enantiomers, is more potent than 3-MF and if you DO resolve, it's only the lower MW of 3-MF that makes it marginally stronger. The thiazole proves to be as potent as the 2-furyl (i.e. twice as potent as the 2-thienyl) and unexpectedly has a duration some x4 longer. I can only presume that the liver enzyme(s) responsible for N-dealkylation have trouble with that aromatic (oxazoles also work but the LogP isn't as favourable). The o-F is just my little joke, 2-fluoroaniline just means 1 more thing to watch but it isn't like more resources appear to actually DO so.
In short, while NPP (N-phenylethyl-4-piperidone) is still the precursor-of-choice (and is watched), 3,3-dimethyl-1-[2-(1,3-thiazol-2-yl)ethyl]piperidin-4-one is an item of commerce. Good luck with the ethyl chloroformate!
It's purely given as an example of just how easy it is to avoid the MoDA and 'watched' precursors. That people aren't even bothering kind of hints that control isn't working AT ALL. That does anger me since fentanyl has become a disaster area with 1000s of people ending up dead from C cut with F. I suspect the authorities aren't really trying very hard.
-----
https://imgur.com/a/JYeLkLt
Mate in 3. I checked with the Home Office and as long as the precursor is intended as a precursor, not as a medicine, I don't even need a licence. OK, it's 3 steps (as opposed to 2 for fentanyl) but that 14-methoxy appears to produce a LOT of delta activity even in the absence of the ether bridge. I'm just pointing out that some REALLY potent stuff is within anyone's reach. The MoDA doesn't cover it! I'm not defending people making this stuff, I'm just appraising people of the fact that it's not exactly difficult to make the stuff and where there is ? to be made, people will.
I admit that the alkylation of the tertiary alcohol is a bit of a pain. Schmidhammer does at least go for an 'uneventful' route but it's and interesting problem. In this case, the absence of certain moieties does make the scaffold more robust (that bridge is a sod for breaking).
-----
https://imgur.com/a/SKCnQFC
One for the training set. It overlays BDPC spectacularly well although Dr. Lednicer had never come across it. So will a m-OH or p-Br work? I'm going to say YES. Will replacing the acetophenone moiety with a different aromatic work. I'm going to say yes. Dimethylaminopivalophenone follows the classic mu training set but like BDPC, this compound shows that you can move that basic nitrogen. I vaguely remember another scaffold with a secondary amine that overlaid BDPC but it had to much sigma affinity. Still, it's all good for your training sets.
Last edited:
S.J.B.
Bluelight Crew
- Joined
- Jan 22, 2011
- Messages
- 6,886
https://imgur.com/a/JYeLkLt
Mate in 3. I checked with the Home Office and as long as the precursor is intended as a precursor, not as a medicine, I don't even need a licence. OK, it's 3 steps (as opposed to 2 for fentanyl) but that 14-methoxy appears to produce a LOT of delta activity even in the absence of the ether bridge.
It's nice that the MoDA doesn't have a blanket ban on morphinans. That said, I'm not sure what uncontrolled commodity chemical would serve as a viable precursor to the compound drawn. I'm curious what you had in mind.
It's nice that the MoDA doesn't have a blanket ban on morphinans. That said, I'm not sure what uncontrolled commodity chemical would serve as a viable precursor to the compound drawn. I'm curious what you had in mind.
Well 1-[2-(furan-2-yl)ethyl]-3,3-dimethylpiperidin-4-one is certainly available. Anyone who knows the Mole people will know exactly where to get it from. I am still amazed at the amount of 2-nitroprop-1-en-1-ylbenzene they still sell. An RT reaction between benzaldehyde & nitroethane with NaOH catalyst is a stroke of genius. Who cares if someone can do it in 2 hours. If the chemist can just fill the reactor at the end of the day, the product is ready for the next day. They have gone a bit off-radar and so I'm guessing they have all the customers they need (how much does a Kg of the nitropropene cost to make?).
The mad major sells just about every ring-substituted aldehyde, nitrostyrene, nitropropene and aryl-methyl ketone known to man. His attempt to distil 3,5-diamino-2,4,6-trinitrobenzaldehyde still makes me laugh. That is his thing. They carefully consulted the MoDA and just ensured that, along with the Poisons Act & the Explosive acts, they weren't breaking the law.
They set me a puzzle that I'm still unable to solve. It's a 1-step route to PMK using only 1 organic compound & 2 inorganic compounds and almost 100% conversion takes place promptly at 64?C (the smell is their right on the button so it happens fast). I think it's a Russian or Chinese paper but I don't think ANYONE ever solved that one.
https://imgur.com/a/L6ZmCBP
Pyeyzolam (known to the Eunoiapharmacopia crew) as explained by OrangeJuice
The Patent Application:
(Journal 6751), GB1813962.6, Applicant: Alcarelle Holdings Limited Title: Mood enhancing compounds. Date Lodged: 28 August 2018
IS this compound. Sadly it only mimics drunk, not a couple of glasses of wine. No problem, my own patents (synth by Ukranian Team) DOES fully replace ethanol.
Would you believe that all of the subjective negative effects of alcohol are mediated by the GABA a1b2d2 subtype and all of the positive effects of alcohol are mediated by the a5b2d2? All of you who tried the 3mf tablets will know that a couple do almost nothing, 8 makes you feel like you imbibed a 1/2 bottle of vodka instantly. So it isn't perfect. Happily I now have a very long-acting ligand (to treat the alcohol dependant) and a thienobenzo that mimics ethanol really well.
I would just like to thank all of the Eunoiapharmacopia crew. Would you believe that isophenidine didn't happen because the shitty chemists removed the H2O thermally so an amine with a low MW wasn't possible. That a RT, fast, cheap route was known was thrown out because they couldn't charge much (given how cheap it was). Truly, in the RC era, people still looked for max profit and diphenidine should never have been distributed. I feel bad about that. I do hope people enjoyed the pynazolam - that was our (me and my family) favourite. We LOVED it.
That the aminorex didn't appear was limited chemistry ability as well.....
Pyeyzolam (known to the Eunoiapharmacopia crew) as explained by OrangeJuice
The Patent Application:
(Journal 6751), GB1813962.6, Applicant: Alcarelle Holdings Limited Title: Mood enhancing compounds. Date Lodged: 28 August 2018
IS this compound. Sadly it only mimics drunk, not a couple of glasses of wine. No problem, my own patents (synth by Ukranian Team) DOES fully replace ethanol.
Would you believe that all of the subjective negative effects of alcohol are mediated by the GABA a1b2d2 subtype and all of the positive effects of alcohol are mediated by the a5b2d2? All of you who tried the 3mf tablets will know that a couple do almost nothing, 8 makes you feel like you imbibed a 1/2 bottle of vodka instantly. So it isn't perfect. Happily I now have a very long-acting ligand (to treat the alcohol dependant) and a thienobenzo that mimics ethanol really well.
I would just like to thank all of the Eunoiapharmacopia crew. Would you believe that isophenidine didn't happen because the shitty chemists removed the H2O thermally so an amine with a low MW wasn't possible. That a RT, fast, cheap route was known was thrown out because they couldn't charge much (given how cheap it was). Truly, in the RC era, people still looked for max profit and diphenidine should never have been distributed. I feel bad about that. I do hope people enjoyed the pynazolam - that was our (me and my family) favourite. We LOVED it.
That the aminorex didn't appear was limited chemistry ability as well.....
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210

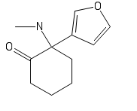
I'll call this new cumpound the D (COC bond and a Ketone; get it?)
The new DCK every disso addict will be boofing. haha (please Don't ban me)
And a little inspiration; I recommand Bird Box (movie about mass psychosis due to entities) and Annihilation, basically TDS stuff but in a movie.
The new DCK every disso addict will be boofing. haha (please Don't ban me)
And a little inspiration; I recommand Bird Box (movie about mass psychosis due to entities) and Annihilation, basically TDS stuff but in a movie.
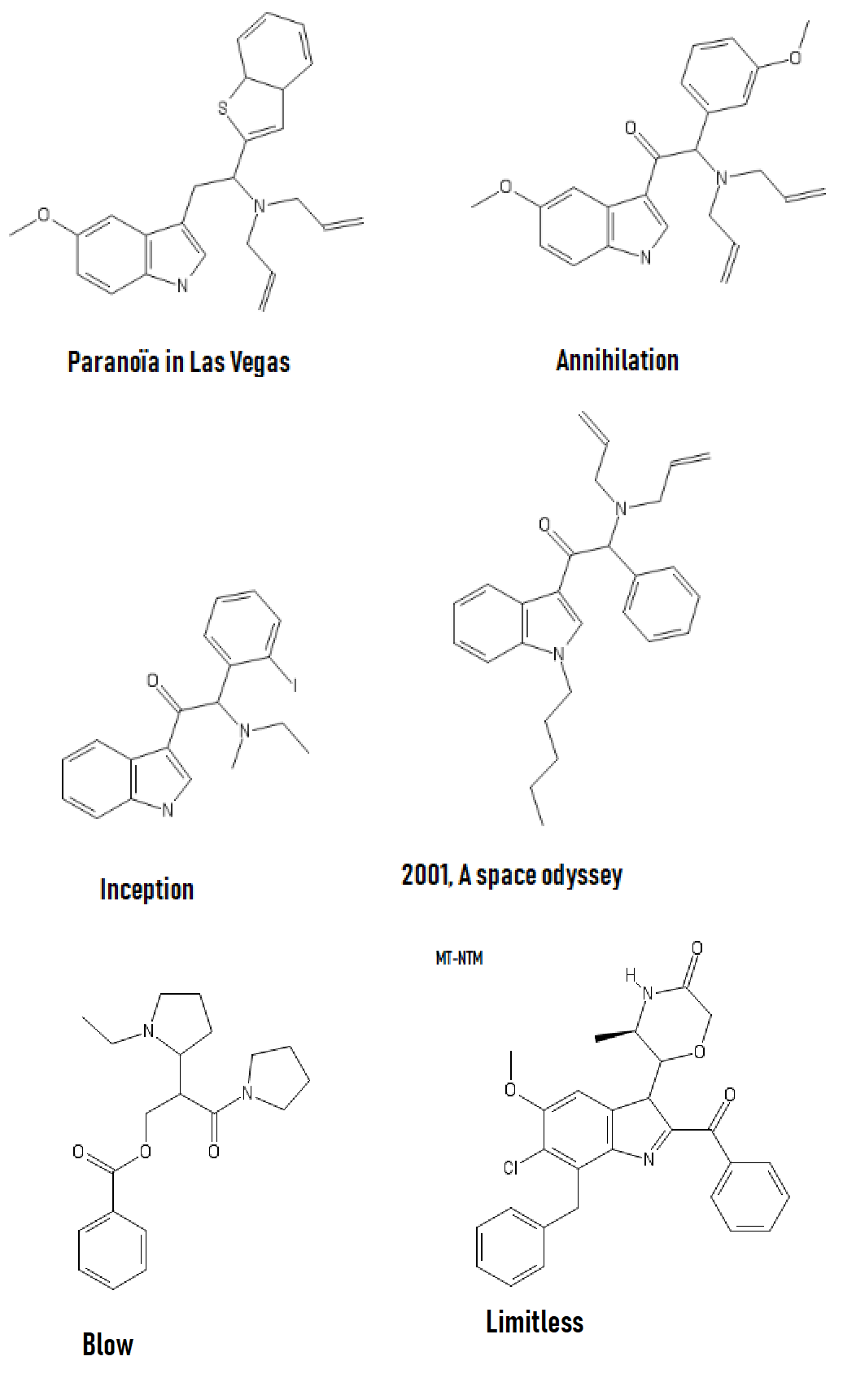
Could someone come up with a NMDA agonist other than those proposed by wiki? Interested by how it lay interact with the consciousness of oneself and its environment.
presumed Deadly toxic, I proudly present
U-47007 (tapent-(tapant = hitting in french (hitman hint))-adol :
presumed Deadly toxic, I proudly present
U-47007 (tapent-(tapant = hitting in french (hitman hint))-adol :
(probably has some HT activity)
Last edited:
Dresden
Bluelighter
- Joined
- Feb 2, 2010
- Messages
- 3,212
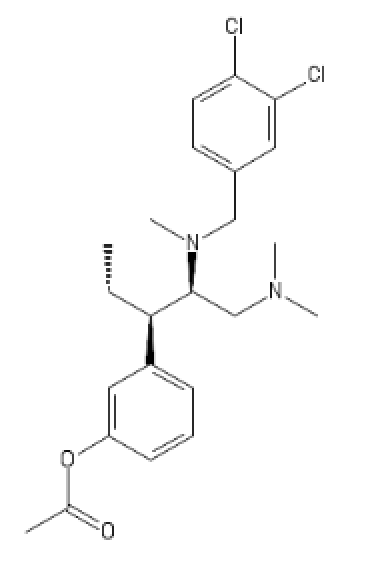
I like D. Maybe add a furanyl chlorine, though?
FOUR TWEENIUS
ANANDA
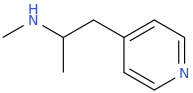
OSIRIS
Great CEVs.
Ah, The Joy Of A Well Placed Propane Pharmacophore!
It's Only Right That You Pay Homage To The Stuff That's About To Blow Up Your Nose. So Just Get On The Floor, And DANCE ALL NIGHT.
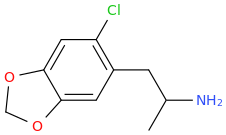
HONDA
Remember, this thread is a "joke." Or Not!

FOUR TWEENIUS

ANANDA

OSIRIS
Great CEVs.
Ah, The Joy Of A Well Placed Propane Pharmacophore!
It's Only Right That You Pay Homage To The Stuff That's About To Blow Up Your Nose. So Just Get On The Floor, And DANCE ALL NIGHT.

HONDA
Remember, this thread is a "joke." Or Not!
Last edited by a moderator:
Dresden has come up with a dew things I actually searched for in Reaxys. Swapping a benzene for an 5-membered O or S containing ring seemed to require the O/S to be ortho - but that doesn't mean that they aren't active. Now, after uncovering 3-(dimethylamino)-2,2-dimethyl-1-phenylpropan-1-one in the 'Annual Report in Medicinal Chemistry (along with nexeridine and a few others), I tried running 3DQSAR data comparing 3,3-dimethyl prodine (ref on here somewhere) and no surprises, it overlays. Then I replaced one of the N-methyl with a 2-phenylethyl moiety and discovered that it overlaid PEPAP. So :
2,2-dimethyl-3-[methyl(2-phenylethyl)amino]-1-phenylpropan-1-one
I commend others to reproduce the results in CHARMM, ChemOffice or similar. Now, the prodine derivative is chiral and while one is x9 M, the other is inactive. Cunningly, in this case, the compound is achiral and far enough, the parent is equipotent. So I'm not going to claim huge activity - likely and order of magnitude higher than M but as you chemists will have noted, it's a single step synthesis. Now I suspect that like related compounds, it causes serious respiratory depression BUT on the plus side, duration is likely to be longer than pethidine or prodine (no ester) so N-demethylation seems the most likely fate.
2,2-dimethyl-3-[methyl(2-phenylethyl)amino]-1-phenylpropan-1-one
I commend others to reproduce the results in CHARMM, ChemOffice or similar. Now, the prodine derivative is chiral and while one is x9 M, the other is inactive. Cunningly, in this case, the compound is achiral and far enough, the parent is equipotent. So I'm not going to claim huge activity - likely and order of magnitude higher than M but as you chemists will have noted, it's a single step synthesis. Now I suspect that like related compounds, it causes serious respiratory depression BUT on the plus side, duration is likely to be longer than pethidine or prodine (no ester) so N-demethylation seems the most likely fate.