Bigazznugz
Bluelighter
- Joined
- Feb 11, 2013
- Messages
- 887
Well this disappeared off the face of the earth after a ton of hype. Not really hype just a long ass wait.
I'm interested what does this mean and how it affects the pharmacology? Is it more reactive because it's charged like that for short period of time? Or does it stay like that?Not only is TFM much bulkier than chlorine, it should also withdraw much more electron density overall via resonance:
.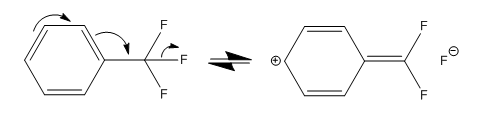
With that + charge delocalized over the ring, as seen in this paper that looked at different substituent effects on aromatic ring reactivity:
pubs.acs.org/doi/abs/10.1021/ja00341a032
Don't know how correct this is since it's been a while since I took organic chemistry, or what this would mean for 2-TFMDCK's activity vs. ketamine, but maybe someone more knowledgeable could offer some insight.
Dude how can you use 14 g in one session that's crazy or did I misunderstand? Do you use low amounts per day?I will be trying this one if it comes my way
I think ketamine is one of the safest, probably second to MXE. The latest ones and 3-MEO-PCP can very easily lead to manic behaviour and a decrease in cognitive functioning in heavy use that can continue into the sober mind for weeks after and triggered worse by other drugs after. They are currently only available to a small audience, if it was like ketamine and people were using it at parties there would be some bad cases.
Ketamine and MXE both have negative physical symptoms. But old BL posts from before 2010 discuss the chronic use of ketamine that was going on when it was available. As long as your exercising, putting lots of food in your stomach and drinking plenty of water when using ketamine and its pure you shouldn't run into too many issues. I've been taking it over 6-7 years now and would use minimum 3g a session more like 14g, I'll piss more often but if I take a break for a few months it calms down. I found MXE still had similar effects in daily use for extended periods (e.g. 2-3 weeks) also. The others I think you'd be loosing the plot a bit before being able to use that long.
the nitrile (CN) shouldn't be hard to reach , right ?
i tried modeling this and the nitrile on iSpartan, and sometimes, i think the nitrile one was aligned. CF3 (TFM) aligned once in a fluke, if i remember correctly, but didn't seem to render with the geometry of ketamine. Also, i guess, they change shape in the blood and fluids and membranes/tissues and all
