fridgebuzz
Bluelighter
Don't usually see many sulfurs in drugs. Once this molecule begins breaking down inside the body, what becomes of it?

N&PD Moderators: Skorpio | someguyontheinternet

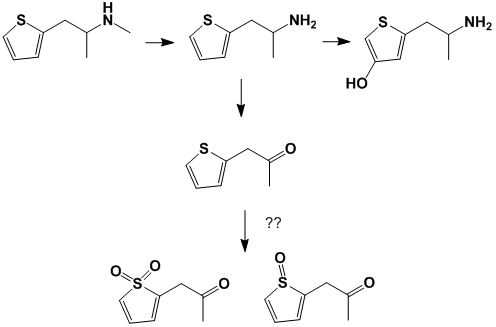
The main metabolite detected in urine in the N-demethylated metabolite nor-MPA,
showing that methiopropamine is only metabolized to a minor extent. The metabolism
includes the cytochrome P450 enzyme CYP2C19 in the liver. In in vitro studies, traces
of methiopropamine hydroxy metabolites could also be detected. However, these
metabolites could not be identified in human urine [11-14].
So 3G, 4G and 5S shouldn't occur at all in humans, considering the intermediates can't form? That fits with what Drgreenthumb posted about metabolite detection in human urine.
