Dresden
Bluelighter
- Joined
- Feb 2, 2010
- Messages
- 3,212
I took a semester of German in college and liked it. I also have a thing for German engineered cars. And I'm a big fan of the trance duo, "Gabriel and Dresden." And I read the chapter in pihkal by the same name which described the German city as being one of he most beautiful in Europe until it and its innocent civilians were brutally firebombed by the US; thus the name was meant to critique the atrocity that is modern warfare. Finally, Dresden is the German equivalent of my real first name, Andrew. Both are derived from the Greek, andros, meaning man or strong. Hope that helps.




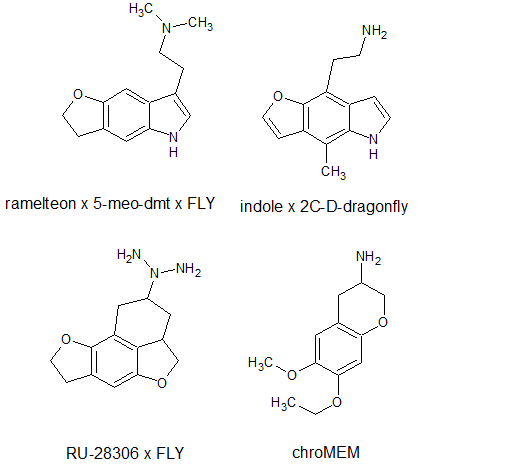
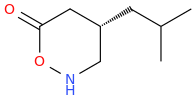
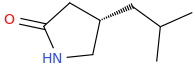
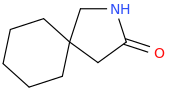
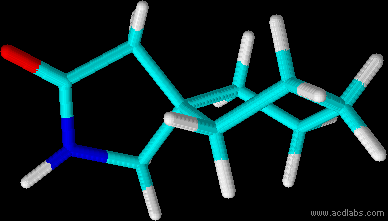


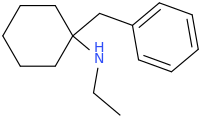
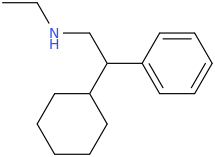
![ethyl[%281-phenylcyclohexyl%29methyl]amine.png](/community/proxy.php?image=http%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2Fethyl%5B%25281-phenylcyclohexyl%2529methyl%5Damine.png&hash=88cded959cee990bf0aa1be89f725567)
![[%281-benzylcyclohexyl%29methyl]%28ethyl%29amine.png](/community/proxy.php?image=http%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2F%5B%25281-benzylcyclohexyl%2529methyl%5D%2528ethyl%2529amine.png&hash=59448c10816758d200ba931d8fb83ea1)
![1'-ethyl-2'%2C4'-dihydro-1'H-spiro[cyclohexane-1%2C3'-quinoline].png](/community/proxy.php?image=http%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2F1%27-ethyl-2%27%252C4%27-dihydro-1%27H-spiro%5Bcyclohexane-1%252C3%27-quinoline%5D.png&hash=c0804aa02746f448dbe25bb993b37fcb)
![1'-ethyl-8'-methoxy-2'%2C4'-dihydro-1'H-spiro[cyclohexane-1%2C3'-quinoline]-2-one.png](/community/proxy.php?image=http%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2F1%27-ethyl-8%27-methoxy-2%27%252C4%27-dihydro-1%27H-spiro%5Bcyclohexane-1%252C3%27-quinoline%5D-2-one.png&hash=23277f00e384eae5069d014d1501f46a)
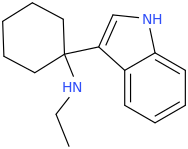
![[cyclohexyl%28phenyl%29methyl]%28ethyl%29amine.png](/community/proxy.php?image=http%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2F%5Bcyclohexyl%2528phenyl%2529methyl%5D%2528ethyl%2529amine.png&hash=c13a9f7dcd463578b61ea24da88df51b)