Roger&Me
Bluelighter
^LOL, nice -- I think the steric bulk at the 4 position would hinder its activity, though.
N&PD Moderators: Skorpio | someguyontheinternet

trans 3-OH C7 acid 4.968
GHB 4.935
trans 3-OH C6 acid 4.921
S-4-Me-GHB (S-GHV) 4.914
GHV 4.912
cis 3-OH C7 acid 4.824
t-HCA 4.719
cis 3-OH C5 acid 4.694
cis 3-OH C6 acid 4.409
trans 3-OH C5 acid 4.395
S-3-Me-GHB 4.382
S-2-Me-GHB 4.331
R-2-Me-GHB 4.328
R-4-Me-GHB (R-GHV) 4.314
R-3-Me-GHB 4.305

Shulgin touches on the piperidine analog of the tryptamine - pip-T - and it seems to be a very strange and malicious compound indeed.
Would this pass the blood-brain barrier? o.o
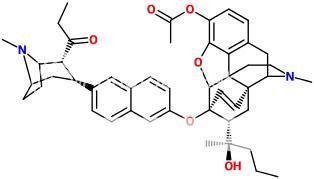
^Beautiful molecule! Has this been bioassayed?

Your point about 5-HT is well taken, but 4-FA ought to cause subjective effects even if it's just releasing 5-HT, shouldn't it? I mean, 5-HT release may antagonize the DAergic effects, but it shouldn't necessarily decrease the overall subjective feeling of being high, right? Unless 5-HT release itself causes little or no subjective effects, while still diminishing the DAergic effects. That would help explain why drugs like MDAI are said to not feel like much by themselves.4-FA is also a serotonin releaser whereas D-amp is not. It's also worthwhile to note that 4-FA cannot be metabolized via p-hydroxylation unlike D-amp.
Does that place a ceiling on the effect of a drug like 4-FA, or can you compensate with higher doses?The 25x preference for DAT for D-amp over 4-FA is the major issue. Lower EC50s at the transporters = more effective as a monoamine releaser = generally more abusable, euphoric, and toxic.
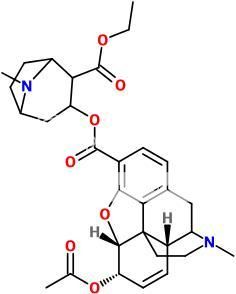
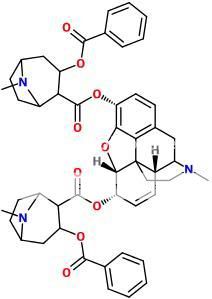
^ 'Speedballamine' lol...