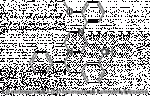morphiquet
Bluelighter
- Joined
- Aug 6, 2005
- Messages
- 128
i came across a new family of opioids some time ago bearing a piperazine-ring in their structure, but i wasn't able to find anything about their potencies or half-lives.they can be distinguished into 3 major classes.
1. 1-(1,2-diphenylethyl)-piperazines
2. 1-cinnamyl-4-propionyl-piperazines
3. 1-((4-amidophenyl)-(phenyl)-methyl)-piperazines
has anyone ever made experiences with those compounds? i'd be very interested to read any information that is related to those compounds.
so don't hesitate to post
1. 1-(1,2-diphenylethyl)-piperazines
2. 1-cinnamyl-4-propionyl-piperazines
3. 1-((4-amidophenyl)-(phenyl)-methyl)-piperazines
has anyone ever made experiences with those compounds? i'd be very interested to read any information that is related to those compounds.
so don't hesitate to post