So i have been looking at Sunifirams (a drug marketted as an ampakine like nootropic) chemical structure lately and i was confused because it is chemically so very similar to benzylpiperazine.
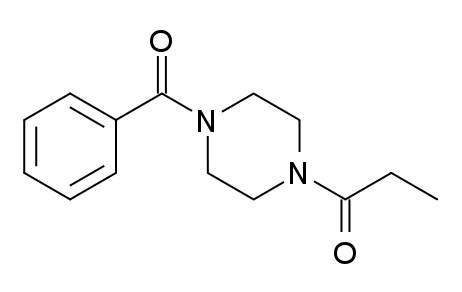 <---Sunifiram
<---Sunifiram
 <---Benzylpiperazine
<---Benzylpiperazine
so basically it is a 4 substituded cathinone analogue of BZP. Shouldnt this chemical be active on DA,NE and Serotonin like other BZP derivates?
Or do BZP Ketones just not work like phenetylamines? (i mean the 4 substitution should not hinder it from beeing active since i guess that one would be metabolized first meaning it should rather increase potency due to longer half life)
Ive read some reports about this nootropic but they dont seem to differentiate sunifiram from other ampakines in terms of stimulant potential.
does anyone have any papers on whether or not it is amphetaminergic active or did anyone ever take this and experience amphetamine-like effects?
would be pretty interesting for me as an adhd person to have an ampakine ndri^^
greetings
M3ph

so basically it is a 4 substituded cathinone analogue of BZP. Shouldnt this chemical be active on DA,NE and Serotonin like other BZP derivates?
Or do BZP Ketones just not work like phenetylamines? (i mean the 4 substitution should not hinder it from beeing active since i guess that one would be metabolized first meaning it should rather increase potency due to longer half life)
Ive read some reports about this nootropic but they dont seem to differentiate sunifiram from other ampakines in terms of stimulant potential.
does anyone have any papers on whether or not it is amphetaminergic active or did anyone ever take this and experience amphetamine-like effects?
would be pretty interesting for me as an adhd person to have an ampakine ndri^^
greetings
M3ph