Hello ADDers,
Through my looking around at various amphetamine analogs I came across this:
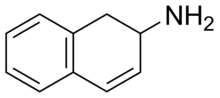
It's pharmacologically active and roughly 1/4 potency of dextroamphetmine. But it's not the activity that I'm interested in, it's the metabolism. I would assume that 2-napthylamine is also pharmacologically active, but it's extreme toxicity/carcinogenity would obviously rule out any use for it.
So my question is, is this likely to metabolise in a much less toxic way, and if so what to? Does the partial saturation of a ring avoid epoxidation?
Through my looking around at various amphetamine analogs I came across this:

It's pharmacologically active and roughly 1/4 potency of dextroamphetmine. But it's not the activity that I'm interested in, it's the metabolism. I would assume that 2-napthylamine is also pharmacologically active, but it's extreme toxicity/carcinogenity would obviously rule out any use for it.
So my question is, is this likely to metabolise in a much less toxic way, and if so what to? Does the partial saturation of a ring avoid epoxidation?
