Valency normally refers to the charge on an ion i.e. how many electrons the neutral atom is missing or has extra. In the HCl form, the amine part of MDMA gains a + charged hydrogen from the H-Cl to give the amine a + charge, which forms an ionic bond with the negative Cl- ion.
I suggest reading some basic organic chemistry regarding
isomers. Pick a book/online page etc that details isomers with good pictures. You don't have to learn it all, but a non in-depth reading will allow you to understand the terminology.
Several different types of isomers exist but not all are relevant to MDMA.
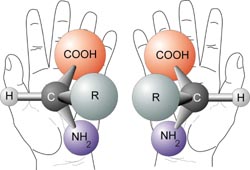
Molecules termed enantiomers, are stereoisomeric forms (isomers) related to each other as are your right and left hands. They are non superimposable images because of the shape resulting from 4 different groups attached to a chiral carbon centre.
Pic from
wiki page on chirality
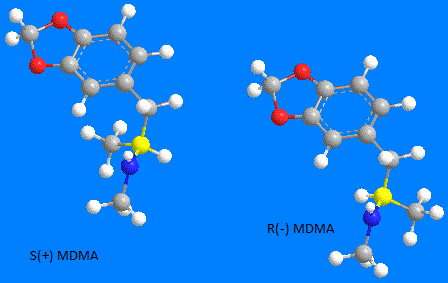
An example of a chirality is the second carbon on the alkyl branch of MDMA (yellow in below pic) that is bonded to:
1) a Nitrogen with 1 Hydrogen & a CH3 ( -NH-CH3 )
2) a Carbon with 3 hydrogens ( -CH3 )
3) a Carbon with 2 Hydrogens ( -CH2- )
4) a Hydrogen (-H )
Spatially, these 4 groups may be positioned differently. Steric repulsion allows by rotation, certain positions to be favoured, so placement of the chiral carbon and the attached groups is not random.
The
l and
d or (-) and (+) designation is arrived at by by passing polarised light through one pure form. Positions of different groups will effect direction and angle of deflection. Determining this direction assigns a character L or (-) for levorotatory (lefthand ), and D or (+) for dextrorotatory ( righthand )
Another type of isomer configuration, resulting in the assignment of an R or S, is determined by priority placement of the groups attached. Viewing the carbon and attached groups as a tetrahedral with the lowest value group at the top, the order of the other three groups (lowest value to highest value priority) determines R for a right hand circular direction and S for a left.
Note that with many molecules, the R or S assignment may exist in either the (-) or (+) from. However with MDMA the (-) is the R form and the (+) is the S form.
Shulgin states as does the CRC handbook on enantiomers, that the S(+) isomer is the most potent empathy producing form in humans, by quite a degree.
Pihkal #109 MDMA
With MDMA, the usual assignments of activity to optical isomers is reversed from all of the known psychedelic drugs. The more potent isomer is the "S" isomer, which is the more potent form of amphetamine and methamphetamine. This was one of the first clear distinctions that was apparent between MDMA and the structurally related psychedelics (where the "R" isomers are the more active).
Most MDMA is (assumed to be) made from the ketone, an achiral group with a double bond to oxygen [ >C=O ] and planer in form. The N (amine)group is attached to C2 via a process known as reductive amination, givng [ >CH-NH-CH3 ]. Several variations of this reaction exist, using different reducing agents or catalysts.
Other methods that arrive at the target via different mechanisms produce a single enantiomer. Isomers can be separated by chromatography and with some enantiomer resolving agents, but it is unlikely this would be done with street intended product.
As to 32 different forms - I would think - not isomers. And although isomers possess different properties, if it ain't an isomer of MDMA it ain't MDMA. It is definitely possible there could be any of 32 different compounds (more even) in pills. Most likely things are binders, substitutes, unreacted precursors, and products of bad lab routine or side reactions occurring during the synthesis.