blase deviant
Bluelighter
- Joined
- May 9, 2004
- Messages
- 2,897
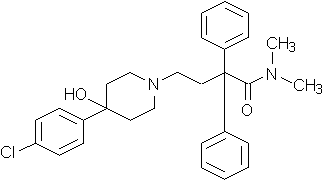
...is it possibly that this will become the next pseudoephedrine/meth situation? Is there anyway to turn this into something viable or is anyone working on it?
Not asking how, just asking if it's being done. Sorry if this is borderline.
Not asking how, just asking if it's being done. Sorry if this is borderline.