Apologies for not responding sooner, visitors and stuff.
I can't find any specific mention in either Qld or NSW relating to legislation but this is hardly surprising as methylone has not yet really grabbed the drug seeking public. It's still much in the early culture phase, if indeed it ever finds acceptance from this market. If and when it does kick off overseas, you can betcha Pete and Bob will ensure legislation is passed BEFORE it fl00ds our streets as a claimed neuroprotective E or something similar. It has certainly been discussed in Forensic Literature.
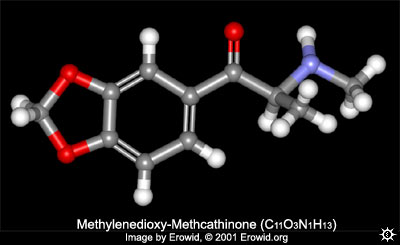
The characterization of some 3,4-methylenedioxycathinone (MDCATH) homologs
Terry A. Dal Cason
Forensic Science International Volume 87, Issue 1, 9-53 (1997)
DOI:10.1016/S0379-0738 (97)02133-6
Abstract
In the past 35 years, a wide variety of illicit drugs have appeared in the clandestine market. Many of these compounds are based on the structure of amphetamine (1-phenyl-2-aminopropane) to which various functional or structural groups have been added. Previous modifications to the amphetamine molecule include addition of a methylenedioxy bridge to give 3,4-methylenedioxyamphetamine, and attachment of alpha,beta-keto oxygen to yield cathinone. A chemical synthesis integrating the salient functional/structural groups of these two classes of amphetamine analogs results in manufacture of methylenedioxycathinone (MDCATH). In each instance, N-alkylation of these analogs provides a series of homologs. Furthermore, many of these analogs/homologs meet several criteria which typically support the clandestine laboratory synthesis of novel illicit drugs (`designer drugs'). The MDCATH analogs represent a potentially new series of `designer drugs' whose chemical characteristics have not previously been reported. Appropriate selection of analytical, chemical and physical tests will enable rapid identification of these analogs by a comparative analysis using the data provided.
Full pdf (Rhodium)
Chem & pharm wise; lets start with explaining briefly what methylone is.
If pseudoephedrine is selected as a starting material for making another psychoactive substance, the usual methods employed involve reducing the -OH group to produce methamphetamine. However, the -OH croup also lends itself to oxidation which produces methcathinone. Reports describe methcathinone as being milder than methamphetamine and a poor substitute.
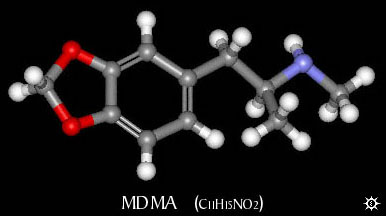
Methylone is to MDMA as Methcathinone is to Methamphetamine. Methylone has all "the bits" of MDMA except for:
On the carbon next to the aromatic ring, 2 Hydrogens present on MDMA have been "exchanged" for an Oxygen.
[it should be said that the chemistry employed in producing these compounds is not quite as simple as the word "exchange" may imply.]
Methylone was was patented by Shulgin and colleagues
Preparation of novel N-substituted-2-amino-3',4'-methylenedioxypropiophenones as anti-depressant and anti-Parkinsonism agents.
Jacob, Peyton, III; Shulgin, Alexander T. (Neurobiological Technologies, Inc., USA).
Patent WO9639133 (1996), 19 pp. CA 126:117961
Abstract
The title compds. [I; R1 = H, C1-6 alkyl, iPr, iBu, sBu, tBu, allyl, propargyl, cyclopropyl; R2 = H], having binding activity for at least one serotonin or dopamine uptake site, and useful for alleviating depression of the central nervous system or symptoms of Parkinson's disease, were prepd. Thus, reaction of methylenedioxybenzene with propionic anhydride in the presence of I2 followed by treatment of the resulting 3,4-methylenedioxypropiophenone with CuBr2 in CH2Cl2, and reaction of 2-bromo-3',4'-methylenedioxypropiophenone with 40% aq. MeNH2 in sulfolane afforded I [R1 = Me; R2 = H]. In general, compds. I are effective at 0.05-10 mg/kg/day.
Methylone has attracted attention due to the fact that claims are made regarding it's similarities to MDMA, yet Methylone inhibits transporter molecules which facilitate uptake of catecholamines
** to membrane receptors, while not interfering with vesicle transporters (inside the cell, responsible for enabling serotonin transport to the axon terminals where it is released from the cell). This results in effectively bypassing the series of mechanisms considered responsible for much MDMA induced neuronal damage.
** Catecholamines = monoamine compounds with 2 x -OH groups on the aromatic ring - eg dopamine, noradrenaline, adrenaline
More on Basics of Catecholamines
European Journal of Pharmacology 381 1999 63–69
Inhibition of plasma membrane monoamine transporters by
b-ketoamphetamines
Nicholas V. Cozzi a,b,), Michael K. Sievert b, Alexander T. Shulgin c, Peyton Jacob III d, Arnold E. Ruoho b
a Department of Pharmacology, East Carolina UniÕersity School of Medicine, GreenÕille, NC 27858, USA
b Department of Pharmacology, UniÕersity of Wisconsin Medical School, Madison, WI 53706, USA
c 1483 Shulgin Road, Lafayette, CA 94549, USA
d Departments of Medicine and Psychiatry, UniÕersity of California-San Francisco, San Francisco, CA 94110, USA
Received 20 May 1999; received in revised form 20 July 1999; accepted 27 July 1999
Abstract
Methcathinone and methylone, the b-ketone analogues of
methamphetamine and 3,4-methylenedioxymethamphetamine MDMA ,
respectively, were tested for neurotransmitter uptake inhibition in vitro.
The b-ketones were threefold less potent than the nonketo drugs at inhibiting platelet serotonin accumulation, with IC ’s of 34.6"4.8 mM and 5.8"0.7 mM, respectively. Methcathinone and 50 methylone were similar in potency to methamphetamine and MDMA at catecholamine transporters individually expressed in transfected glial cells. For dopamine uptake, IC ’s were 0.36"0.06 mM and 0.82"0.17 mM, respectively; for noradrenaline uptake, IC values 50 50 were 0.51"0.10 mM and 1.2"0.1 mM, respectively.
In chromaffin granules, IC ’s for serotonin accumulation were 112"8.0 mM for 50 methcathinone and 166"12 mM for methylone, 10-fold higher than the respective values for methamphetamine and MDMA. Our results indicate that methcathinone and methylone potently inhibit plasma membrane catecholamine transporters but only weakly inhibit the vesicle transporter.
Abstract
As for nomenclature; The IUPAC naming is probably something like
N, alpha-Dimethyl-1,3-benzodioxole-5-ethanamine-1-one. Shulgin tends to use a more traditional approach to nomenclature (although his abbreviations are his own naming system) 2-methylamino-1-(3,4-methylenedioxyphenyl)propan-1-one is easier to visualise IMO.
VeloxideX, the compounds you mentioned:
3,4-Methylenedioxy-phenyl-2-propanone is also known as Piperonal methyl ketone, 3,4 MD-phenyl-acetone etc., is the precursor normally used to make MDXX. It is very tightly scheduled, and although has applications in the perfume industry, possession of it would require appropriate permits and involve End User Declaration for any purchase.
1-(3-methoxy-4,5-methylenedioxyphenyl)-2-propanamine is MMDA or the amphetamine made from
Myristicin, an allyl benzene differing to safrole by the addition of a methoxy group - next to the methylenedioxy group -on the aromatic ring. Myristicin is sourced in nature from Myristica Fragrans (nutmg, mace), parsley or dill oil.
This compound was scheduled long before MDMA. It was known about and experimented with during the sixties. As metabolism of myristicin also produces MMDA via methylation in the liver, the practice of eating large quantities of nutmeg once attracted a wide interest. That is until you've tried the trip..

I've got some old psychiatric stuff on MMDA somewhere. If I can find it I'll post any relevant info.
As usual, indebted to; Erowid, Rhodium and the bee place.
[Edit: changed IUPAC naming after thinking about it; p_d]