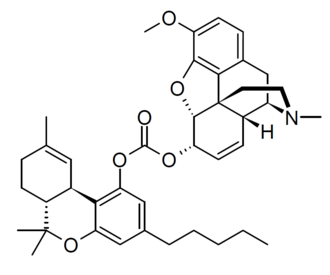
6,14-ethenomorphinans are semisynthetic opiate derivatives containing an ethylene bridge between positions 6 and 14 in ring-C of the morphine skeleton that imparts a rigid molecular structure. These compounds represent an important family of opioid receptor ...

www.ncbi.nlm.nih.gov
The mystery of
6,14-Endoethenotetrahydrooripavine.
The above link is a deep dive showing just how complicated the synthesis and isolation of etorphine actually IS. The truth is, it's unlikely to replace any of the black marked high-potency opioids soon.
But let's consider the first step. Thebaine (or in some cases oripavine) are reacted with a
dienophile
noun di·eno·phile
dīˈenəˌfīl
plural-s
definition:
the olefinic or acetylenic component (such as maleic anhydride) that is seeking a diene in the Diels-Alder reaction.
Now it's well known that 1,3-butandiene and ethene can be reacted to produce cyclohexene. There are MANY examples of this class of reaction being used to produce polymers. So one would think that the mystery compound would be a major product of oripavine and ethene. Not the ONLY product mind you because depending on the face being attacked, the -CH=C- bond could end up above or below the C-ring.
Now I actually DON'T think this is a major issue since hydrogenation yields the dihydro derivatives that appear to be uniformly more potent.
So potentially,, a compound listed as being some x400 morphine in potency is just two steps from oripavine (which isn't mentioned in the UNODC list of controlled drugs and seeminly isn't a controlled precursor.
So IF I had to guess, I would guess that...
(6
R)-11hydroxy-15-methoxy-5-methyl-13-oxa-5-azahexacyclo[13.2.2.12,8.01,6.02,14.012,20]icosa-8(20),9,11-triene
AKA
6,14-hexahydrooripavine
WILL be a reasonable candidate.
614H