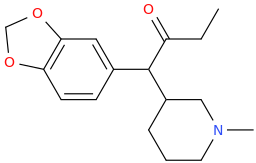
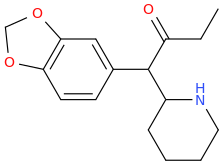
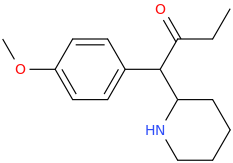
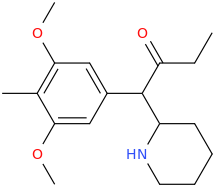
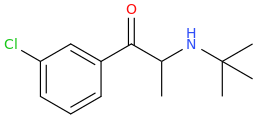
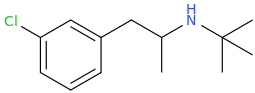
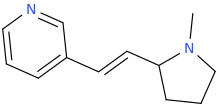
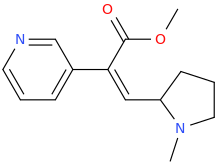
THE_REAL_NITTY_GRITTY
(E)-1-(pyridin-3-yl)-2-(1-methyl-2-pyrrolidinyl)ethene
TASTY_WORLD
(Z)-1-(pyridin-3-yl)-2-(1-methyl-2-pyrrolidinyl)-1-carbomethoxyethene
NOBLESSE_OBLIGE
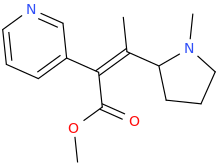
(Z)-1-(pyridin-3-yl)-2-(1-methyl-2-pyrrolidinyl)-1-carbomethoxy-2-methylethene
UNDER_SIEGE
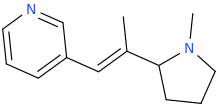
(E)-1-(pyridin-3-yl)-2-(1-methyl-2-pyrrolidinyl)-2-methylethene
TWIGGY
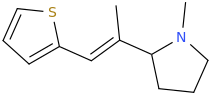
(E)-1-(thiophen-2-yl)-2-(1-methyl-2-pyrrolidinyl)-2-methylethene
BRIDGETTE_BARDO
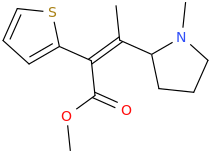
(E)-1-(thiophen-2-yl)-2-(1-methyl-2-pyrrolidinyl)-2-methyl-1-carbomethoxyethene
AL_CAPONE
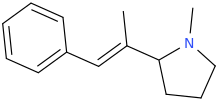
(E)-1-phenyl-2-(1-methyl-2-pyrrolidinyl)-2-methyl-1-ethene
OWSLEY
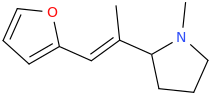
(E)-1-(furan-2-yl)-2-(1-methyl-2-pyrrolidinyl)-2-methyl-1-ethene
MADONNA
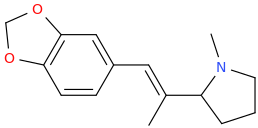
(E)-1-(3,4-methylenedioxyphenyl)-2-(1-methyl-2-pyrrolidinyl)-2-methyl-1-ethene
BRITTNEY_SPEARS
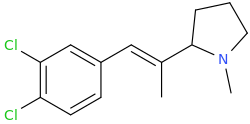
(E)-1-(3,4-dichlorophenyl)-2-(1-methyl-2-pyrrolidinyl)-2-methyl-1-ethene
EMINEM
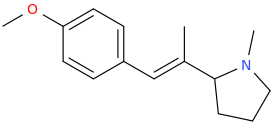
(E)-1-(4-methoxyphenyl)-2-(1-methyl-2-pyrrolidinyl)-2-methyl-1-ethene