Fertile
Bluelighter
- Joined
- Mar 31, 2022
- Messages
- 1,627
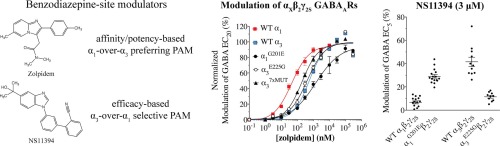
The 3 commonly known 'Z-drugs' are a1 selective hypnotics with a short duration of action but of the 3, Zolpidem has been the most well researched. Indeed necopidem (sedative/anxiolytic), saripidem (sedative/anxiolytic) & alpidem (anxiolytic) all od which received market licences in some nations. They also provide a useful QSAR to define duration and GABAa selectivity.
 www.proquest.com
www.proquest.com
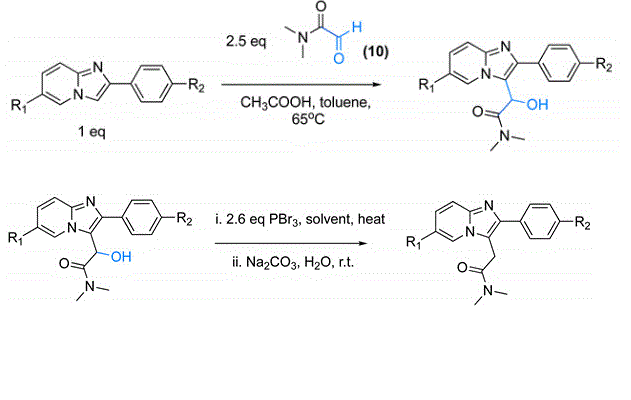
The key to producing one of these compounds as an RC is the complexity of the synthesis, the cost of the initial precursors and the potency of the final product.
Possibly the most interesting is the research compound pagoclone. While working with Professor Nutt I was able to acquire a sample of pagoclone ant it certainly has alcohol-like activity. But it's dose-response curve means that past a certain (quite low) dose, the response curve plateaus and so while it's excellent at emulating 2 glasses of wine, it has no effect beyond that point. Still there are patents showing that the structure giving partial a1,a3,a4 & a5 activity can be altered for produce pure PAMs i.e. an RC that will make the user 'fall over' drunk... without actually falling over, throwing up, suffer fluctuating mood or amnesia... the negative effects associated with alcohol.
I'm going to sit down and find and link to every patent that refers to these non-benzodiazepine anxiolytic/hypnotic compounds.
While it's arguable as to the reach of the DEAs scheduling of certain benzodiazepines as schedule 1 and what that means. Sooner or later producers are going to require a compound as active as bromazolam (for example) but isn't under any legal control.
So please bare with me. This is going to take many weeks.
Structure/activity relationship studies at the benzodiazepine receptor: Pyrido (3,2-b) indoles and annelated 1,4-benzodiazepines as putative BZR ligands - ProQuest
Explore millions of resources from scholarly journals, books, newspapers, videos and more, on the ProQuest Platform.
The key to producing one of these compounds as an RC is the complexity of the synthesis, the cost of the initial precursors and the potency of the final product.
Possibly the most interesting is the research compound pagoclone. While working with Professor Nutt I was able to acquire a sample of pagoclone ant it certainly has alcohol-like activity. But it's dose-response curve means that past a certain (quite low) dose, the response curve plateaus and so while it's excellent at emulating 2 glasses of wine, it has no effect beyond that point. Still there are patents showing that the structure giving partial a1,a3,a4 & a5 activity can be altered for produce pure PAMs i.e. an RC that will make the user 'fall over' drunk... without actually falling over, throwing up, suffer fluctuating mood or amnesia... the negative effects associated with alcohol.
I'm going to sit down and find and link to every patent that refers to these non-benzodiazepine anxiolytic/hypnotic compounds.
While it's arguable as to the reach of the DEAs scheduling of certain benzodiazepines as schedule 1 and what that means. Sooner or later producers are going to require a compound as active as bromazolam (for example) but isn't under any legal control.
So please bare with me. This is going to take many weeks.