-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Ketamine salts solubility
- Thread starter fastandbulbous
- Start date
- Status
- Not open for further replies.
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 6,990

BUDDY
(4-bromo-2,5-dimethoxyphenylmethyl)piperazine

AMBER
(4-ethyl-2,5-dimethoxyphenylmethyl)piperazine

DUSTIN HOFFMAN LA ROCHE
1-(4,7-dimethoxyindan-5-yl)-2-aminopropane

ELLE
1-(benzofuran-6-yl)-2-ethylaminopropane

BEAU
1-(1-thiaindole-6-yl)-2-aminopropane
Everything I Do Is Simple.

DARRELL
1-(1-oxa-2-oxoindane-6-yl)-2-methylaminopropane

DARRELL METABOLITE
1-(3-hydroxy-4-(carboxymethyl)phenyl)-2-methylaminopropane
Note similarity to GHB. (Metabolizes into a GHB-ish moiety.)

FIREBALL
N-((2-hydroxy)benzyl)-2-(3,4-methylenedioxyphenyl)-1-methylethylamine

TRIPP
N-methyl-1,4-dimethoxy-3-bromomorphinan
Trippy Opiate?
We Can Feel That Rhythm, Moving Through Your System.

SMACK ATTACK
N-methyl-3,4-methylenedioxy-6-ethylmorphinan
In Xanadu Did Kubla Khan Decree A Stately Pleasure Dome To Be.

KUBLY BEAR (KB)
N-ethyl-3,4-methylenedioxy-6-ethyl-5,7-di-oxomorphinan

IODOMESCALINE
1-(3,4,5-trimethoxy-2-iodophenyl)-2-aminoethane
^--designed to be qualitatively similar to mescaline yet also more potent by molar comparison
Last edited:
Congrats
Nagelfar
Bluelight Crew
New reagent discovered from CFC waste
I thought the chemists here would find this interesting.
The closest I got to working with CFCs (besides getting lifetime CFC handling certification in the state of Washington for union hall port jobs) was once experimenting with Du Pont brand Freon as an inhalant.
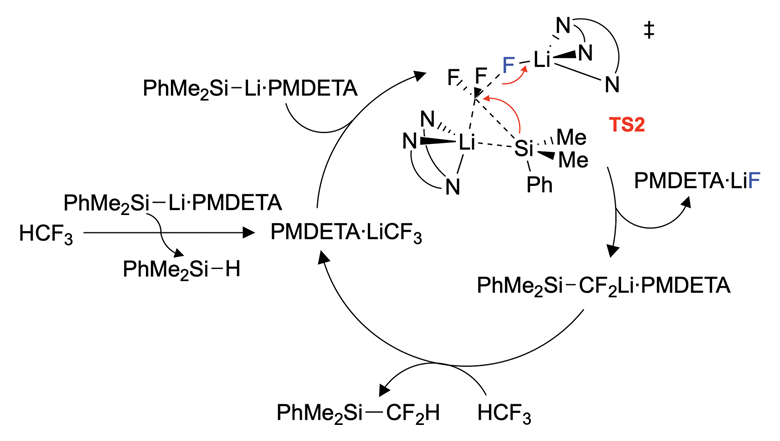
An image showing the proposed reaction cycle for trifluoromethane defluorosilyation.
Source: © Mark Crimmin/Imperial College London
I thought the chemists here would find this interesting.
The closest I got to working with CFCs (besides getting lifetime CFC handling certification in the state of Washington for union hall port jobs) was once experimenting with Du Pont brand Freon as an inhalant.

An image showing the proposed reaction cycle for trifluoromethane defluorosilyation.
Source: © Mark Crimmin/Imperial College London
Valuable difluoromethylating agent obtained from refrigerant waste
BY HAYLEY RUSSELL8 OCTOBER 2020
UK-based researchers have devised a way to turn waste trifluoromethane into a useful reagent for introducing fluorinated building blocks into drug compounds.
Trifluoromethane (HCF3) is a waste product formed in the production of chlorofluorocarbon refrigerant gases. It is also a greenhouse gas with a global warming potential 11,700 times higher than CO2. As it poses such a high environmental risk and has few applications, it is either stored or destroyed.
Now, researchers led by Mark Crimmin at Imperial College London have developed a method for converting trifluoromethane into a reagent that can insert difluoromethyl (CF2H) groups into molecules during pharmaceutical synthesis.
Difluoromethyl groups are present in many pharmaceutical and agrochemical compounds. Scientists add C–F bonds to pharmaceutical compounds to increase drug stability and solubility. Existing methods for synthesising the difluoromethylating agent PhMe2SiCF2H use HCF2Cl, which has since been banned.
The team has proposed a reaction cycle for the defluorosilylation of trifluoromethane
Crimmin’s team has shown a simple silyl lithium reagent can break the strong C–F bond in trifluoromethane at room temperature with the reaction completing within five minutes. Researchers from Japan have demonstrated a similar reaction previously where they react the equivalent boryl lithium reagent with HCF3. They proposed a mechanism but did not test it.
The team at Imperial College conducted a mechanistic study. They say that this was the hardest part of the research, as the reaction was too fast for traditional kinetics techniques. To overcome this, the researchers turned to computational studies to gain better insight into the mechanism. Their calculations support a mechanism in which the silicon reagent seems to be acting as both a catalytic base and a nucleophile. CF3H first undergoes a deprotonation to form a trifluoromethanide intermediate, which is then susceptible to direct nucleophilic attack.
‘This type of reaction would generally require expensive or ozone depleting sources such as HCF2Cl to synthesise the R3SiCF2H reagent, for which more sustainable solutions are needed,’ explains Tatiana Besset, an organofluorine researcher at the University of Rouen in France. ‘But it’s very interesting to see how a greenhouse gas and a fluorinated byproduct coming from industry waste, was used to generate a highly valuable nucleophilic difluoromethylating source.’
‘We are looking to scale up the synthesis beyond the gram-scale,’ says Crimmin. ‘We have some concerns about potential safety issues here, as the reactions are exothermic, and so plan to use flow chemistry to do this.’ Crimmin says they also have plans to collaborate with another research group to investigate radiolabelling in the reaction.
References
1. D J Sheldon, G Coates and M R Crimmin, Chem. Commun., 2020, DOI: 10.1039/d0cc04592f (This article is open access.)
2. S Ito, N Kato and K Mikami, Chem. Commun., 2017, 53, 5546 (DOI: 10.1039/c7cc02327h)
Last edited:
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 6,990
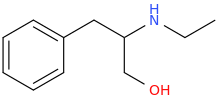
FAIRNYMPH
1-phenyl-2-ethylamino-3-hydroxypropane
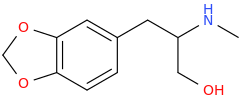
PHREEX (Dave)
1-(3,4-methylenedioxyphenyl)-2-methylamino-3-hydroxypropane
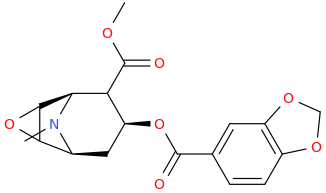
BMP (BOLIVIAN MARCHING POWDER)
4-carbomethoxy-6,7-epoxytropan-3α-oxycarbonyl-3,4-methylenedioxybenzene
Last edited:
Nagelfar
Bluelight Crew
These days machine learning is taking a steep curve proportional to how deep they can go.
This, however, is the Pandora's box & tool kit for the novices floating around our neck of the web:
Universal chemistry software can turn words into chemicals
This, however, is the Pandora's box & tool kit for the novices floating around our neck of the web:
Universal chemistry software can turn words into chemicals
Universal chemistry software can turn words into chemicals
BY TOM METCALFE13 OCTOBER 2020
A robotic system that can read the chemical literature, turn it into working reaction pathways and use them to automatically create specific chemicals has been developed at the University of Glasgow.
The researchers say the system is the first ‘universal’ architecture for automated chemical synthesis, and can be implemented on any capable robot to turn methods in a book or journal into the desired product. It’s already been tested on common synthesis methods, and the automated manufacture of several test compounds, including the analgesic lidocaine, the Dess–Martin oxidation reagent periodinane and the fluorinating agent AlkylFluor.1
We’ve invented the CPU [central processing unit] for chemistry
LEE CRONIN, UNIVERSITY OF GLASGOW
Lee Cronin, who leads the team behind the work, says the system is independent of robot hardware and is a breakthrough in creating a universal language for chemical methods. It’s based on a markup language that can represent the varied steps of chemical methods, called XDL – where the X is the Greek letter chi and comes from χημία, the Greek word for chemistry.
XDL will be open source and free to use, like the HTML used on webpages. It has rules for about 44 different synthesis operations to make almost any known molecule, and can be extended to recognise further operations, he says.
A scheme showing the universal system for the automatic execution of chemical synthesis from the literature
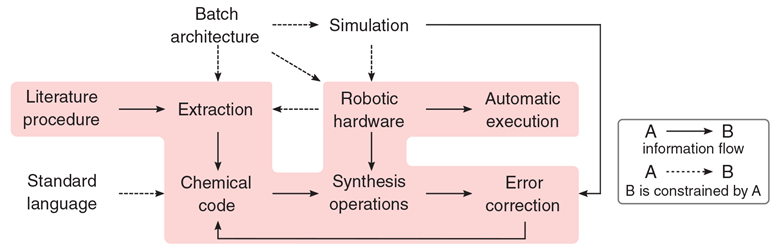
Source: © Science/AAAS
The workflow of the universal system that can translate the chemistry literature into a synthesis
‘We’ve invented the CPU [central processing unit] for chemistry,’ he says. ‘That’s really important right now, because all the chemistry robots in the world are not only expensive, but they can’t be programed in the same way.’
The new system fixes that by describing chemical methods in XDL, and then using the XDL descriptions to generate instructions for a virtual machine – a theoretical device dubbed a ‘ChemPU’. They can then be run on different robots through software interpreters for the virtual ChemPU instructions, instead of creating customised steps for any particular chemistry robot. ‘This eliminates all that work,’ Cronin says.
Universal chemistry
Cronin’s team has developed the prototype on one of his fume hood-sized chemputers – there are now 12 of them with different configurations in his lab, automatically working on several projects. The next stage of the project will be to implement the system on chemistry robots at other institutions, Cronin says.
The system has three stages: the first uses a scanner to read pages from the chemistry literature – a synthesis method in a book or a journal, say – and parses it into XDL, with error correction to let a human chemist make fixes or adjustments. ‘That’s important, because then you can make sure that your robot doesn’t just halt and catch fire,’ he says.
The next stage turns the XDL into instructions for the ChemPU virtual machine, and the last stage interprets the ChemPU instructions for any given chemistry robot. The system will generate descriptive errors if a robot’s hardware is not up to the task, he says. The markup language XDL is integrated into a software development environment that can combine methods from the literature with input from a human chemist.
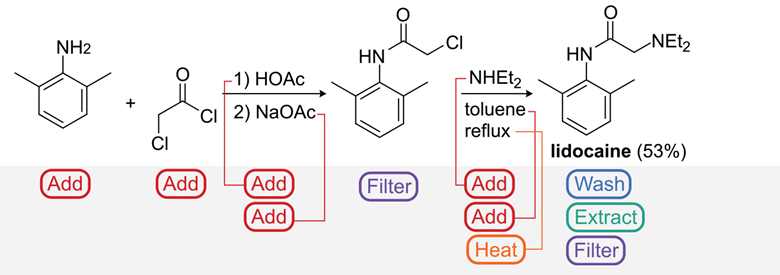
An image showing the lidocaine synthesis
Source: © Science/AAAS
The steps the universal system planned and performed to produce lidocaine
‘Take your favourite cocktail … vodka Martini or something … and write it down,’ Cronin says. ‘The system will translate it into what reactor to use, where the pipes are, what the temperature is, the stirring rate and all that stuff.’
Cronin doesn’t see chemistry robots replacing humans, however. Instead, they will let chemists get on with important tasks, while leaving time-consuming, boring or dangerous work to robots. ‘It’s going to allow chemists to make many more molecules than they could ever have dreamed of before and discover many more drugs, because they can focus on what they are good at,’ he says.
IBM Zurich’s Teandro Laino, a researcher on RoboRXN, notes that Cronin’s system uses rules to process the chemical literature, while their system uses a method based on natural language processing (NLP). Such rule-based approaches could be hard to improve without extensive human intervention, and might prove weaker than advanced NLP methods, he adds.
But Cronin counters that the XDL rules are open, editable and ensure the scientific validity of a system to connect methods from literature with their implementations on any chemistry robot – including the proprietary robot of the RoboRXN system.
Martin Burke of the University of Illinois at Urbana–Champaign, who specialises in organic synthesis and chemical innovation, says the new system is part of a brave new world of ‘democratised’ chemistry. The paper shows the tremendous potential to revolutionise chemistry by strategically merging computer science with automated organic synthesis, he says.
References
S H M Mehr et al, Science, 2020, DOI: 10.1126/science.abc2986
Shady's Fox
Bluelighter
make the chemical automatically
waw
i think 3d printing it's bettah
ha
nah
ok
waw
i think 3d printing it's bettah
ha
nah
ok
Nagelfar
Bluelight Crew
Dopamine & Serotonin unexpected effects.
Dopamine, serotonin involved in sub-second perception, cognition.
In first-of-their-kind observations in the human brain, an international team of researchers has revealed two well-known neurochemicals — dopamine and serotonin — are at work at sub-second speeds to shape how people perceive the world and take action based on their perception.
The discovery shows researchers can continually and simultaneously measure the activity of both dopamine and serotonin — whose receptor and uptake sites are therapeutic targets for disorders ranging from depression to Parkinson’s disease — in the human brain.
Furthermore, the neurochemicals appear to integrate people’s perceptions of the world with their actions, indicating dopamine and serotonin have far more expansive roles in the human nervous system than previously known.

Carbon Fiber Microelectrodes
Virginia Tech researchers with the Fralin Biomedical Research Institute Center for Human Neuroscience Research construct carbon fiber microelectrodes for real-time detection of dopamine and serotonin activity in human patients. Credit: Virginia Tech
Known as neuromodulators, dopamine and serotonin have traditionally been linked to reward processing — how good or how bad people perceive an outcome to be after taking an action.
The study published in the journal Neuron on October 12, 2020, opens the door to a deeper understanding of an expanded role for these systems and their roles in human health.
“An enormous number of people throughout the world are taking pharmaceutical compounds to perturb the dopamine and serotonin transmitter systems to change their behavior and mental health,” said P. Read Montague, senior author of the study and a professor and director of the Center for Human Neuroscience Research and the Human Neuroimaging Laboratory at the Fralin Biomedical Research Institute at Virginia Tech Carilion. “For the first time, moment-to-moment activity in these systems has been measured and determined to be involved in perception and cognitive capacities. These neurotransmitters are simultaneously acting and integrating activity across vastly different time and space scales than anyone expected.”
Better understanding of the underlying actions of dopamine and serotonin during perception and decision-making could deliver important insight into psychiatric and neurological disorders, the researchers said.
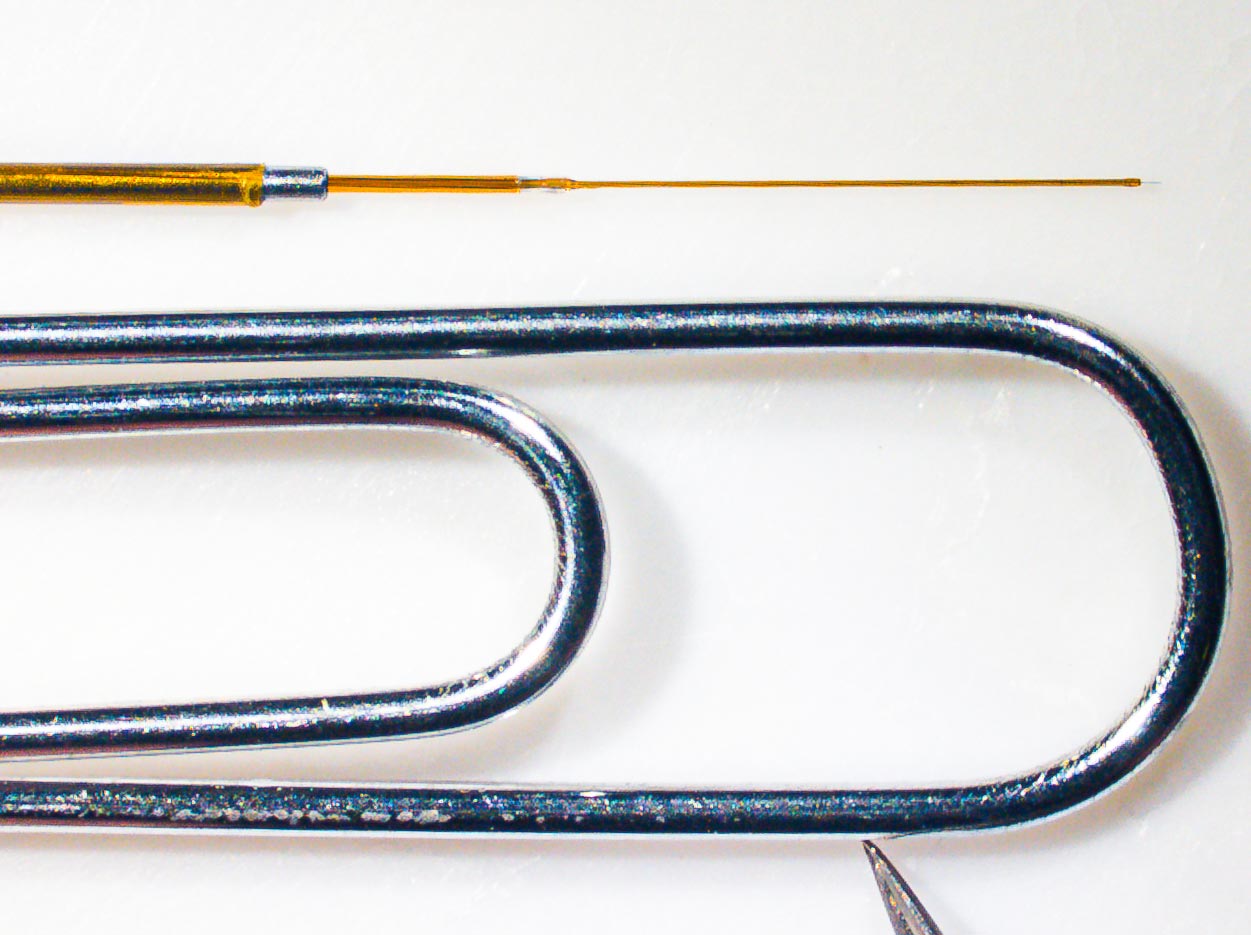
Carbon Fiber Microelectrode Size Comparison
The relative size of a microelectrode used to make recordings of dopamine and serotonin activity during deep brain stimulation procedures. Credit: Virginia Tech
“Every choice that someone executes involves taking in information, interpreting that information, and making decisions about what they perceived,” said Kenneth Kishida, a corresponding author of the study and an assistant professor of physiology and pharmacology, and neurosurgery, at Wake Forest School of Medicine. “There’s a whole host of psychiatric conditions and neurological disorders where that process is altered in the patients, and dopamine and serotonin are prime suspects.”
Lack of chemically specific methods to study neuromodulation in humans at fast time scales has impeded understanding of these systems, according to Montague, who is an honorary professor at the Wellcome Center for Human Neuroimaging at University College London and a professor of physics at the Virginia Tech College of Science.
But now, in first-ever measurements, scientists used an electrochemical method called “fast scan cyclic voltammetry,” which employs a small carbon fiber microelectrode that has low voltages ramped across it for real-time detection of dopamine and serotonin activity.
In the study, researchers recorded fluctuations in dopamine and serotonin using specially designed electrodes in five patients undergoing deep brain stimulation electrode implantation surgery to treat essential tremor or Parkinson’s disease. Patients were awake during surgery, playing a computer game designed to quantify aspects of thought and behavior while the measurements were taken.

Read Montague, a computational neuroscientist at the Center for Human Neuroscience Research at Virginia Tech’s Fralin Biomedical Research Institute, said dopamine and serotonin are at work at sub-second speeds to shape how people perceive the world and take action based on their perception. Credit: Virginia Tech
On each round of the game, patients briefly viewed a cloud of dots and were asked to judge the direction they were moving. The method, designed by corresponding author Dan Bang, a Sir Henry Wellcome Postdoctoral Fellow, and Steve Fleming, a Sir Henry Dale/Royal Society Fellow, both at the Wellcome Center for Human Neuroimaging at University College London, helped indicate that dopamine and serotonin were involved in simple perceptual decisions, outside of the traditional context of rewards and losses.
“These neuromodulators play a much broader role in supporting human behavior and thought, and in particular they are involved in how we process the outside world,” Bang said. “For example, if you move through a room and the lights are off, you move differently because you’re uncertain about where objects are. Our work suggests these neuromodulators — serotonin in particular — are playing a role in signaling how uncertain we are about the outside environment.”
Montague and Kishida, along with Terry Lohrenz, a research assistant professor, and Jason White, a senior research associate, now both at the Fralin Biomedical Research Institute, started working on a new statistical approach to identify dopamine and serotonin signals while still at the Baylor College of Medicine in Houston, Texas.
“Ken rose to the challenge of doing fast neurochemistry in human beings during active cognition,” Montague said. “A lot of other good groups of scientists were not able to do it. Aside from the computation of enormous amounts of data, there are complicated issues to solve, including great, fundamental algorithmic tasks.”
Until recently, only slow methodologies such as PET scanning could measure the impact of neurotransmitters, but they were nowhere near the frequency or volume of the second-to-second measurements of fast scan cyclic voltammetry.
The measurements in the new study were taken at the Wake Forest Baptist Medical Center, and involved neurosurgical teams led by Adrian W. Laxton and Stephen B. Tatter.
“The enthusiasm the neurosurgeons have for this research is derived from the same reasons that drove them to be doctors — first and foremost, they want to do the best for their patients, and they have a real passion for understanding how the brain works to improve patient outcomes,” said Kishida, who oversaw the data collection in the operating room during the surgeries. “Both are collaborative scientists along with Charles Branch, the chair of the neurosurgery department at Wake Forest, who has been an amazing advocate for this work.”
Likewise, Montague said, “You can’t do it without the surgeons being real, shoulder-to-shoulder partners, and certainly not without the people who let you make recordings from their brains while they are having electrodes implanted to alleviate the symptoms of a neurological disorder.”
Montague had read a study in the Proceedings of the National Academy of Sciences that prompted him to approach colleagues Bang and Fleming at University College London to tailor a task for patients to perform during surgery that would reveal sub-second dopamine and serotonin signaling in real-time inference about the external world – separate from their often-reported roles in reward-related processes.
“I said I have this new method to measure dopamine and serotonin, but I need you to help with the task,” Montague said. “They ended up in the study. The research really took a lot of hard work and an integrated a constellation of people to obtain these results.”
Reference: “Sub-second Dopamine and Serotonin Signaling in Human Striatum during Perceptual Decision-Making” by Dan Bang, Kenneth T. Kishida, Terry Lohrenz, Jason P. White, Adrian W. Laxton, Stephen B. Tatter, Stephen M. Fleming and P. Read Montague, 12 October 2020, Neuron.
DOI: 10.1016/j.neuron.2020.09.015
The research was funded by grants to various researchers from the Wellcome Trust, the National Institutes of Health including the National Institute on Drug Abuse, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke.
polymath
Bluelight Crew
Looks quite futuristic... Automated peptide synthesizers have existed for over 20 years, but in that case there are not many precursors and reagents needed for building almost any linear peptide chain from natural amino acids.
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 6,990

ANDREA
1-phenyl-1-carbomethoxy-2-aminoethane

CHADWICK BOSEMAN
N,N-diethyl-12-oxonia-Lysergamide
I Wouldn't Take That If I Were You.

DEAN
1-(indole-3-yl)-2-methylaminopropane

EDNA
1-(indole-3-yl)-2-ethylaminopropane

BEN
1-(indole-3-yl)-1-oxo-2-aminopropane

BEAU
2-(3-methoxyphenyl)-2-aminocyclohexane
Last edited:
Honestly the big hurdle for a lot of clandestine chemistry is getting the reagents. My only chemistry experience was a perfunctory organic chemistry lab in undergrad (where we actually did the lidocaine synthesis from above), and I feel like it would not be super hard to synthesize many common recreational drugs given a suitable lab and the precursors and enough time to read the literature.
(okay I would be fairly scared doing a grignard my first time or like a cyanogen bromide cyclization, but protocol and routine usually vanquisg fear or at least shove it into a corner where it appears in the form of caution).
The things keeping me from doing this are 1) my fear of legal repercussions, 2) my lack of a safe lab space 3) the difficulty of acquiring precursors, (which can lead to the time consuming search for legal pre precursors or even pre-pre precursors).
In my eyes this machine takes out the lack of a lab space. I would still have to source the chemicals as they are consumable, and I feel that would be fairly difficult without enlisting some Gordon Todd Skinner unsavory type.
(okay I would be fairly scared doing a grignard my first time or like a cyanogen bromide cyclization, but protocol and routine usually vanquisg fear or at least shove it into a corner where it appears in the form of caution).
The things keeping me from doing this are 1) my fear of legal repercussions, 2) my lack of a safe lab space 3) the difficulty of acquiring precursors, (which can lead to the time consuming search for legal pre precursors or even pre-pre precursors).
In my eyes this machine takes out the lack of a lab space. I would still have to source the chemicals as they are consumable, and I feel that would be fairly difficult without enlisting some Gordon Todd Skinner unsavory type.
Pretty much. If you have the reagents, the glassware is going to be easier and cheaper to procure than a fancy robot.Honestly the big hurdle for a lot of clandestine chemistry is getting the reagents.
Flynnal
Bluelighter
- Joined
- Aug 14, 2012
- Messages
- 859
Nice work. I'd love to know whether I respond to SSRIs or not...perhaps if I had low serotonin that they would benefit me. I'd be willing to take an SSRI in spite of the likelihood of sexual dysfunction if it meant that I would never have to feel depressed ever again. But in all honesty I would prefer a satisfactory sexual function...which upsets me because these drugs are the mainstay of depression treatment.
If my problem is dopamine deficiency, well, we have stuff for that, they're called amphetamines.
If my problem is dopamine deficiency, well, we have stuff for that, they're called amphetamines.
Nagelfar
Bluelight Crew
I live in Washington state in the U.S., and here state insurance available to low income, for free, covers a genetics test called GeneSight. It checks to see specifically what genes relate to SSRIs, what interactions, even what serotonin receptor polymorphisms you have.
In my case I have a unusual genotype and only Selegiline & Pristiq were indicated for me, Pristiq: a drug state insurance wouldn't normally cover, but because of that test, my prescriber was able to get permission from the state insurance company to prescribe it to me at no cost, not even co-pay.
I think other states in the U.S. do the same thing, but they won't mention it, you have to bring it up to them.
In my case I have a unusual genotype and only Selegiline & Pristiq were indicated for me, Pristiq: a drug state insurance wouldn't normally cover, but because of that test, my prescriber was able to get permission from the state insurance company to prescribe it to me at no cost, not even co-pay.
I think other states in the U.S. do the same thing, but they won't mention it, you have to bring it up to them.
interesting. can medicaid cover it? ill check their website, i assume its genesight.com and ill ask em. but i guess it differs by state so we shall see.
but anyway, how reliable is it really? this can be a game changer, honestly. wouldnt it change the whole antidepressant industry when you know what you should take instead of having pyschiatrists prescribe you whatever they feel like??
but anyway, how reliable is it really? this can be a game changer, honestly. wouldnt it change the whole antidepressant industry when you know what you should take instead of having pyschiatrists prescribe you whatever they feel like??
- Status
- Not open for further replies.











