-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Ketamine salts solubility
- Thread starter fastandbulbous
- Start date
- Status
- Not open for further replies.
4meSM
Bluelighter
- Joined
- Jan 6, 2014
- Messages
- 3,181
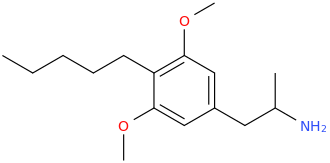
VISHNU
dextro-2-methylamino-1-phenylpropane
I'd offer myself as a guinea pig to test this new substance, it's a dirty job but someone has to do it... You might be onto something with this Vishnu shit.
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
4meSM
Bluelighter
- Joined
- Jan 6, 2014
- Messages
- 3,181
Cool.
But if I understand it correctly it's actually a peptide that kills his "host" (the same bacteria producing it).
I thought it tracked and destroyed other identical peptide molecules, maybe it does that too? It's not clear to me.
The title instantly made me think of prions which actually turn healthy versions of the prion protein into malignant/toxic versions just by coming into contact with them.
But if I understand it correctly it's actually a peptide that kills his "host" (the same bacteria producing it).
I thought it tracked and destroyed other identical peptide molecules, maybe it does that too? It's not clear to me.
The title instantly made me think of prions which actually turn healthy versions of the prion protein into malignant/toxic versions just by coming into contact with them.
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,502

PEGGY
1-(4-methoxycyclohex-1-yl)-2-aminopropane

ALEX
1-(4-methoxycyclohex-1-yl)-2-methylaminopropane

NEGROGESIC
1-(4-methoxycyclohex-1-yl)-1-oxo-2-methylaminopropane

STEPHANIE
1-(4-methoxycyclohex-1-yl)-2-ethylaminopropane

ETHAN
1-(2,5-dioxo-4-bromocyclohex-1-yl)-2-aminoethane

FITZ
1-(4-ethyl-2,5-dioxocyclohex-1-yl)-2-aminopropane

CASSANDRA
1-(4-ethyl-2,5-dioxocyclohex-1-yl)-2-ethylaminopropane

CASSIOPEIA
1-(4-ethyl-2,5-dioxocyclohex-1-yl)-1-oxo-2-ethylaminopropane

MICHELLE
1-(3,5-dioxo-4-ethylcyclohex-1-yl)-2-aminopropane

ERIN
1-(3,4,5-trioxocyclohex-1-yl)-2-aminoethane

BRENDA
1-(3,4,5-trioxocyclohex-1-yl)-2-aminopropane

JAMES (anesthesia)
2,6-diisopropylcyclohexanone

PEA
2-phenyl-1-aminoethane
Two to 3 grams gave me great CEVs.
Because it did.
Last edited:
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
A new bacterial molecule with the unsavory tendency to track down and kill others of its own kind has been discovered in the human microbiome by researchers at Princeton's Department of Chemistry. Named Streptosactin, it is the first small molecule found to exhibit fratricidal activity.
The discovery by the Seyedsayamdost lab is detailed in the Journal of the American Chemical Society (JACS).
The research describes a veritable needle-in-a-haystack hunt in which streptosactin was located "at the edge of detection." Its production is so minimal and so difficult to track that researchers achieved the results only through a complex interplay of factors.
They used a clever bioinformatic search strategy developed a few years ago by Leah Bushin, a sixth-year graduate student in the Seyedsayamdost lab. This "genome-first" approach allowed them to screen molecules for two key characteristics: community behavior (thus uncovering the fratricide), and structural or topological novelty.
The researchers then used advanced mass spectrometry tactics to separate out the signal from noise, manipulating and concentrating the culture extracts over a thousand-fold before locating the sought-after compound after months of searching.
The Cain-and-Abel behavior has been found in another bacterium where enzymes or large proteins provide the fratricidal activity—but never before in a small molecule. The purpose of the behavior is not fully understood. Researchers believe fratricide could contribute to evolutionary fitness by enhancing the genetic makeup of the sibling population.
"It's crazy, and I was surprised by it, too. This bioactivity is something we would never have predicted," said Mohammad Seyedsayamdost, associate professor of chemistry and associated faculty in molecular biology. "But that's how microbes live—there is no morality. It's just raw survival.
"Fratricide is good for the fitness or strength of this species," he added. "It's one way in which this bacterium generates a diverse genetic makeup, so it can increase the chances of surviving any challenge or condition it encounters."
An assist from genome sequencing
The lab's research opens a new window onto the vast, largely unexplored microbiome, that aggregate of microorganisms that lives in the mammalian body and likely plays a significant role in the health of all mammals. So uncharted is the microbiome that it has been described as a frontier every bit as exotic as the surface of the moon or the bottom of the ocean.
Princeton lab discovers small "Cain-and-Abel" molecule
The Princeton University Department of Chemistry lab members who discovered Streptosactin: Leah Bushin, Mohammad Seyedsayamdost, and Brett Covington. Credit: C. Todd Reichart
Natural products chemists like those in the Princeton lab try to discover and understand the molecules that microbes use to interact with their environments. Those interactions allow them to work together, to acquire food material, to compete, even to kill each other. Scientists then use this information to generate drug leads for antibiotics and antivirals; in fact, some 70% of all antibiotics we use clinically come from these sources.
"Natural product research can be really frustrating because luck plays a significant role. In a way it's a little like fishing," said Bushin, the lead author on the paper Discovery and Biosynthesis of Streptosactin, a Sactipeptide with an Alternative Topology Encoded by Commensal Bacteria in the Human Microbiome, which appears in JACS this week.
"After months of searching, you think you have a hit and then you go through the process of characterizing it—is it new? Is it known? If it isn't, you have to start from square one. But when you do find something new like streptosactin, it's this rush of adrenaline that's unbelievable. That's why you go through the months of hard work. As a scientist, it's definitely what gets me into the lab every day."
Mass Spectrometry Delivers
Even with a new search approach and an extremely sensitive instrument, streptosactin was difficult to identify from live cultures; its concentration was measured in the picomolar range.
After bioinformatic prediction and characterization of the biosynthetic enzymes, Brett Covington, a postdoctoral researcher in the Seyedsayamdost lab, managed to pinpoint the molecule in bacterial culture extracts. He cultured the organism "in a medium that it likes," and used clever mass spectrometry tactics to locate the chemical fingerprints he was looking for.
"The problem we had with streptosactin is that, in lab cultures, it's not produced well at all. We have a really sensitive instrument and still, it's barely detectible over the noise," said Covington. "So for months I was looking at things, asking could they be noise or could they be real?
"It's a roller coaster with natural products chemistry," Covington added. "If we had a little bit worse of an instrument, we wouldn't have found it. If we didn't concentrate it as well, we wouldn't have found it. If we didn't have the right types of methods that we were using, we wouldn't have seen it. You had to know exactly what you were looking for and you had to look for a really long time."
Bushin said, "I truly believe Brett is one of only a few people in the world who could have completed this task."
The properties and behavior of streptosactin have yet to be puzzled through: what is its role in the microbiome? Why the fratricidal behavior? And what other discoveries remain from the more than 600 natural products predicted by Bushin's search algorithm? These will form the basis of future research projects spearheaded by the Seyedsayamdost lab.
"There are multiple these projects that can be supported by this work," said Seyedsayamdost.
More information: Leah B. Bushin et al. Discovery and Biosynthesis of Streptosactin, a Sactipeptide with an Alternative Topology Encoded by Commensal Bacteria in the Human Microbiome, Journal of the American Chemical Society (2020). DOI: 10.1021/jacs.0c05546
- Joined
- May 11, 2011
- Messages
- 3,427
This next paper is possibly the best ketamine paper I have read to this date (no more k holing sheep).
Deep posteromedial cortical rhythm in dissociation
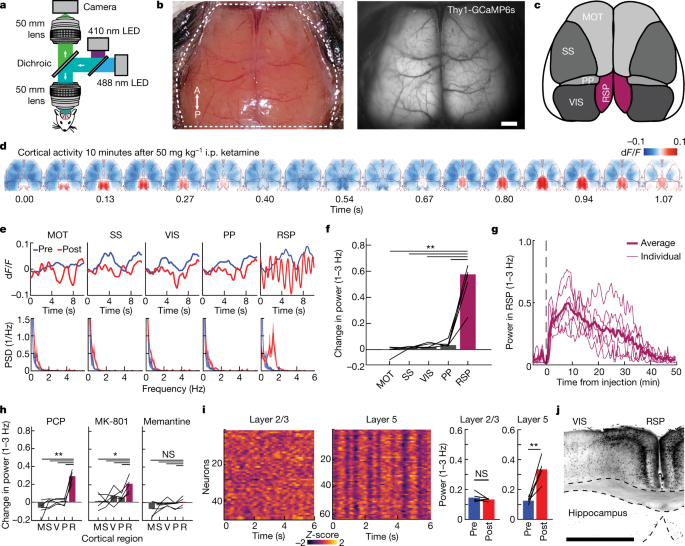
Advanced imaging methods now allow cell-type-specific recording of neural activity across the mammalian brain, potentially enabling the exploration of how brain-wide dynamical patterns give rise to complex behavioural states1,2,3,4,5,6,7,8,9,10,11,12. Dissociation is an altered behavioural state in which the integrity of experience is disrupted, resulting in reproducible cognitive phenomena including the dissociation of stimulus detection from stimulus-related affective responses. Dissociation can occur as a result of trauma, epilepsy or dissociative drug use13,14, but despite its substantial basic and clinical importance, the underlying neurophysiology of this state is unknown. Here we establish such a dissociation-like state in mice, induced by precisely-dosed administration of ketamine or phencyclidine. Large-scale imaging of neural activity revealed that these dissociative agents elicited a 1–3-Hz rhythm in layer 5 neurons of the retrosplenial cortex. Electrophysiological recording with four simultaneously deployed high-density probes revealed rhythmic coupling of the retrosplenial cortex with anatomically connected components of thalamus circuitry, but uncoupling from most other brain regions was observed—including a notable inverse correlation with frontally projecting thalamic nuclei. In testing for causal significance, we found that rhythmic optogenetic activation of retrosplenial cortex layer 5 neurons recapitulated dissociation-like behavioural effects. Local retrosplenial hyperpolarization-activated cyclic-nucleotide-gated potassium channel 1 (HCN1) pacemakers were required for systemic ketamine to induce this rhythm and to elicit dissociation-like behavioural effects. In a patient with focal epilepsy, simultaneous intracranial stereoencephalography recordings from across the brain revealed a similarly localized rhythm in the homologous deep posteromedial cortex that was temporally correlated with pre-seizure self-reported dissociation, and local brief electrical stimulation of this region elicited dissociative experiences. These results identify the molecular, cellular and physiological properties of a conserved deep posteromedial cortical rhythm that underlies states of dissociation.
 www.nature.com
www.nature.com
The tldr by figure:
1: using brain imaging 3 hz oscillations of firing are identified in the retrosplenial cortex during high dose ketamine.
2: these waves are characterized electrophisiologically.
3: these oscillations map strongly to measures of dissociation. They are able to use optogenetics to create these waves of firing with light stimulation and they cause dissociation (3b has some of the strongest evidence I have seen of the "plateau" effect commonly cited with dxm).
4: they identify 2 sets of ion channels that drive the effect.. Nmda receptors (fairly obvious but a good internal positive control) and hcn channels which provide a pacemaker current. They abolish the effects by performing global knockouts of the channels, and then using a cre driven system, restoring the channels solely in the retrosplenial cortex.
5: finally they drop the fucking mic.
They found a patient who had seizures that cause dissociative effects, and was previously implanted with electrodes in their brain for mapping of their seizures . They found the seizures induce rhythmic activity in the retrosplenial cortex.
They briefly stimulated this person's brain and were able to induce dissociative experiences.
This is such a rare thing in neuroscience, and for it to cap off such a methodical and elegant paper is amazing. I have been popping off at my mates for like the last hour after reading this.
Definately read the paper it's a nature paper so pretty clear.
Deep posteromedial cortical rhythm in dissociation
- Sam Vesuna,
- Isaac V. Kauvar,
- Ethan Richman,
- Felicity Gore,
- Tomiko Oskotsky,
- Clara Sava-Segal,
- Liqun Luo,
- Robert C. Malenka,
- Jaimie M. Henderson,
- Paul Nuyujukian,
- Josef Parvizi &
- Karl Deisseroth
- -Show fewer authors
- 341 Altmetric
- Metricsdetails
Advanced imaging methods now allow cell-type-specific recording of neural activity across the mammalian brain, potentially enabling the exploration of how brain-wide dynamical patterns give rise to complex behavioural states1,2,3,4,5,6,7,8,9,10,11,12. Dissociation is an altered behavioural state in which the integrity of experience is disrupted, resulting in reproducible cognitive phenomena including the dissociation of stimulus detection from stimulus-related affective responses. Dissociation can occur as a result of trauma, epilepsy or dissociative drug use13,14, but despite its substantial basic and clinical importance, the underlying neurophysiology of this state is unknown. Here we establish such a dissociation-like state in mice, induced by precisely-dosed administration of ketamine or phencyclidine. Large-scale imaging of neural activity revealed that these dissociative agents elicited a 1–3-Hz rhythm in layer 5 neurons of the retrosplenial cortex. Electrophysiological recording with four simultaneously deployed high-density probes revealed rhythmic coupling of the retrosplenial cortex with anatomically connected components of thalamus circuitry, but uncoupling from most other brain regions was observed—including a notable inverse correlation with frontally projecting thalamic nuclei. In testing for causal significance, we found that rhythmic optogenetic activation of retrosplenial cortex layer 5 neurons recapitulated dissociation-like behavioural effects. Local retrosplenial hyperpolarization-activated cyclic-nucleotide-gated potassium channel 1 (HCN1) pacemakers were required for systemic ketamine to induce this rhythm and to elicit dissociation-like behavioural effects. In a patient with focal epilepsy, simultaneous intracranial stereoencephalography recordings from across the brain revealed a similarly localized rhythm in the homologous deep posteromedial cortex that was temporally correlated with pre-seizure self-reported dissociation, and local brief electrical stimulation of this region elicited dissociative experiences. These results identify the molecular, cellular and physiological properties of a conserved deep posteromedial cortical rhythm that underlies states of dissociation.

Deep posteromedial cortical rhythm in dissociation - Nature
Dissociative states in mouse and human brains are traced to low-frequency rhythmic neural activity—with distinct molecular, cellular and physiological properties—in the deep retrosplenial cortex and the posteromedial cortex.
The tldr by figure:
1: using brain imaging 3 hz oscillations of firing are identified in the retrosplenial cortex during high dose ketamine.
2: these waves are characterized electrophisiologically.
3: these oscillations map strongly to measures of dissociation. They are able to use optogenetics to create these waves of firing with light stimulation and they cause dissociation (3b has some of the strongest evidence I have seen of the "plateau" effect commonly cited with dxm).
4: they identify 2 sets of ion channels that drive the effect.. Nmda receptors (fairly obvious but a good internal positive control) and hcn channels which provide a pacemaker current. They abolish the effects by performing global knockouts of the channels, and then using a cre driven system, restoring the channels solely in the retrosplenial cortex.
5: finally they drop the fucking mic.
They found a patient who had seizures that cause dissociative effects, and was previously implanted with electrodes in their brain for mapping of their seizures . They found the seizures induce rhythmic activity in the retrosplenial cortex.
They briefly stimulated this person's brain and were able to induce dissociative experiences.
This is such a rare thing in neuroscience, and for it to cap off such a methodical and elegant paper is amazing. I have been popping off at my mates for like the last hour after reading this.
Definately read the paper it's a nature paper so pretty clear.
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,502

MARGARET
1-(3,4-dichloro-2,5-dimethoxyphenyl)-2-aminopropane

HAO LI
1-(3,4-dibromo-2,5-dimethoxyphenyl)-2-aminopropane

ANDY C CANDY
1-(3,4-diiodo-2,5-dimethoxyphenyl)-2-aminopropane
You Need Candy.

ELTON DAVID BAILEY
1-(3,4-difluoro-2,5-dimethoxyphenyl)-2-aminopropane
You Need Fairies.
Last edited:
Cortexiphan
Bluelighter
- Joined
- Aug 25, 2011
- Messages
- 99
Structure of a Hallucinogen-Activated Gq-Coupled 5-HT2A Serotonin Receptor
Bryan Roth, David Nichols et al. is making real strides to elucidate the true mechanism of hallucinogens. Paper of the year!
https://www.cell.com/cell/fulltext/S0092-8674(20)31066-7?utm_medium=homepage
Hallucinogens like lysergic acid diethylamide (LSD), psilocybin, and substituted N-benzyl phenylalkylamines are widely used recreationally with psilocybin being considered as a therapeutic for many neuropsychiatric disorders including depression, anxiety, and substance abuse. How psychedelics mediate their actions—both therapeutic and hallucinogenic—are not understood, although activation of the 5-HT2A serotonin receptor (HTR2A) is key. To gain molecular insights into psychedelic actions, we determined the active-state structure of HTR2A bound to 25-CN-NBOH—a prototypical hallucinogen—in complex with an engineered Gαq heterotrimer by cryoelectron microscopy (cryo-EM). We also obtained the X-ray crystal structures of HTR2A complexed with the arrestin-biased ligand LSD or the inverse agonist methiothepin. Comparisons of these structures reveal determinants responsible for HTR2A-Gαq protein interactions as well as the conformational rearrangements involved in active-state transitions. Given the potential therapeutic actions of hallucinogens, these findings could accelerate the discovery of more selective drugs for the treatment of a variety of neuropsychiatric disorders.
Bryan Roth, David Nichols et al. is making real strides to elucidate the true mechanism of hallucinogens. Paper of the year!
https://www.cell.com/cell/fulltext/S0092-8674(20)31066-7?utm_medium=homepage
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,502

TONI MORRISSON
7-methoxy-1,3-dihydro-1-ethyl-5-(2-chlorophenyl)-1,4-benzodiazepine-2-one
And chill.

HODOR
7,8-methylenedioxy-1,3-dihydro-1-methyl-5-(2-fluorophenyl)-1,4-benzodiazepin-2-one

JULIE ANNA HANNAH
7-methylthio-1,3-dihydro-1-methyl-5-phenyl-1,4-benzodiazepin-2-one

OPRAH DAVID WINPHREEX
4-(3,4-methylenedioxyphenyl)-1-(1-methyl-3-(4-methoxyphenyl)-3-oxopropyl)-piperidine
haldol analogue
Last edited:
Ballz_Trippington
Bluelighter
- Joined
- Sep 18, 2013
- Messages
- 1,293
EDIT: THAT EXTRA N on the benzene ring makes it different from DMT?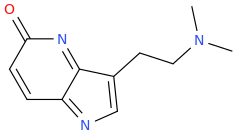
PATTY
1-(5-oxo-4-azaindole-3-yl)-2-dimethylaminoethane
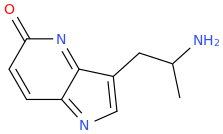
JEB
1-(5-oxo-4-azaindole-3-yl)-2-aminopropane
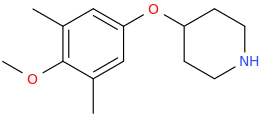
AZRAEL777
piperidin-4-yl 3,5-dimethyl-4-
methoxyphenyl ether
That 5oxo-Dmt looks rather ininteresting ....wonder if thst would be active and if so as a psychedelic...makes me sender oubout 4oxo-dmt.
Fascinating work Rectify!!!!
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,502
Thank you!
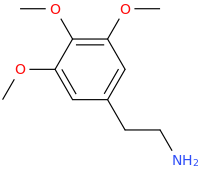
KRISHNA
1-(3,4,5-trimethoxyphenyl)-2-aminopropane

SHIVAYA
1-(3,4,5-trichlorophenyl)-2-aminopropane

ANN SYDNEY HARDEE CATENA SHULGIN
1-(3,4,5-tribromophenyl)-2-ethylaminopropane
Want To See Some Faces? Try THAT--^.
Pareidolia

LE JUNK
dipropylamino-2-(5-methoxy-2-p-ethoxybenzoyl-1,3-benzimidazole-1-yl)ethane

KRISHNA
1-(3,4,5-trimethoxyphenyl)-2-aminopropane

SHIVAYA
1-(3,4,5-trichlorophenyl)-2-aminopropane

ANN SYDNEY HARDEE CATENA SHULGIN
1-(3,4,5-tribromophenyl)-2-ethylaminopropane
Want To See Some Faces? Try THAT--^.
Pareidolia

LE JUNK
dipropylamino-2-(5-methoxy-2-p-ethoxybenzoyl-1,3-benzimidazole-1-yl)ethane
Last edited:
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210

Screenshot-20200920-202129-2 hosted at ImgBB
Image Screenshot-20200920-202129-2 hosted in ImgBB
MFPEP
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
Take a look at these :
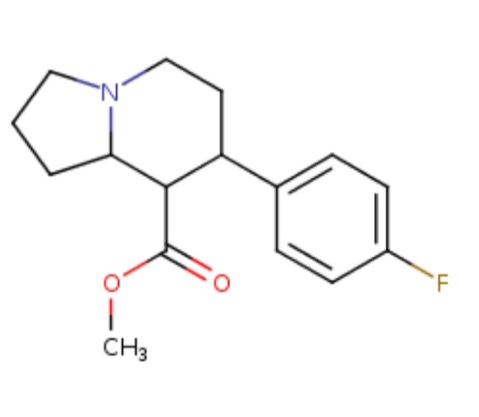
FACTO DEUS
4'Fluoro-8-Carbomethoxy-7-PhenylOctahydroIndolizine
Party sweetener.
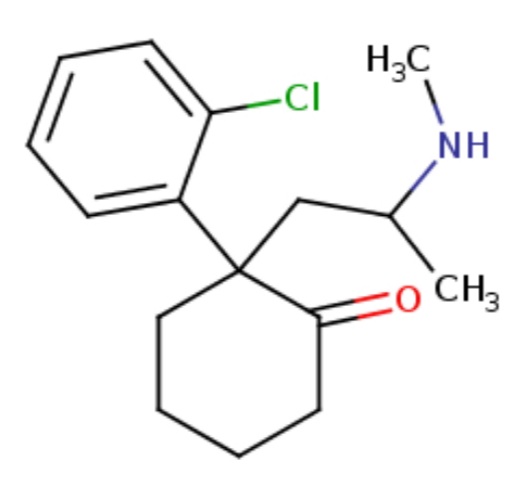
SPEEDY KATE
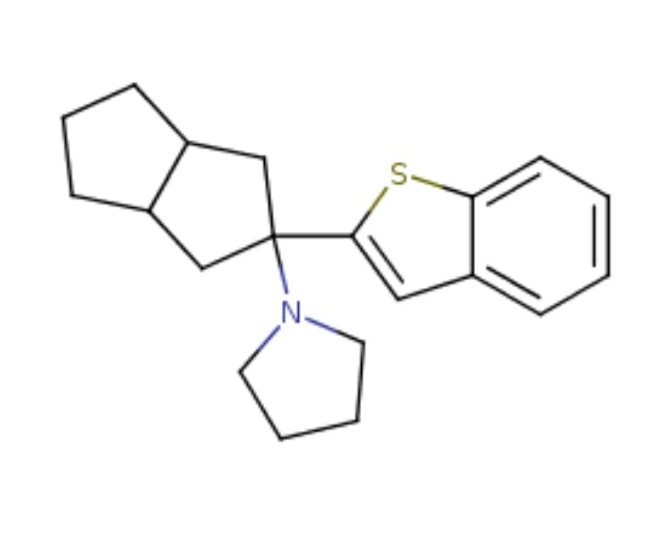
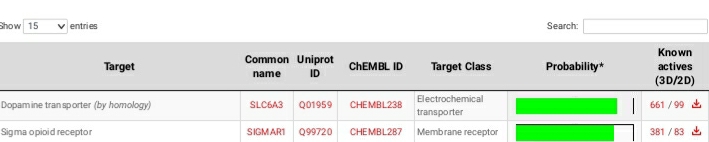
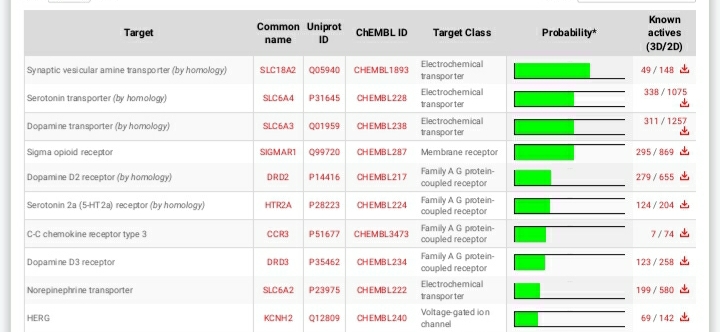
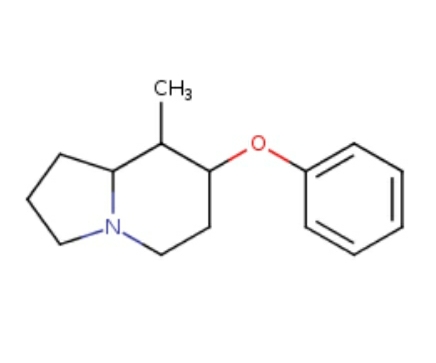
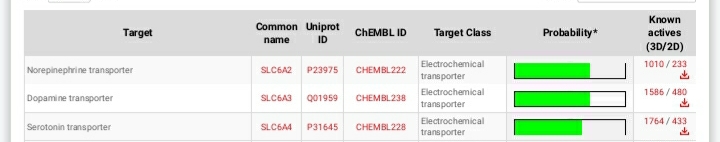
Shorter acting version of MEPHOZINE
All these are dopamimetic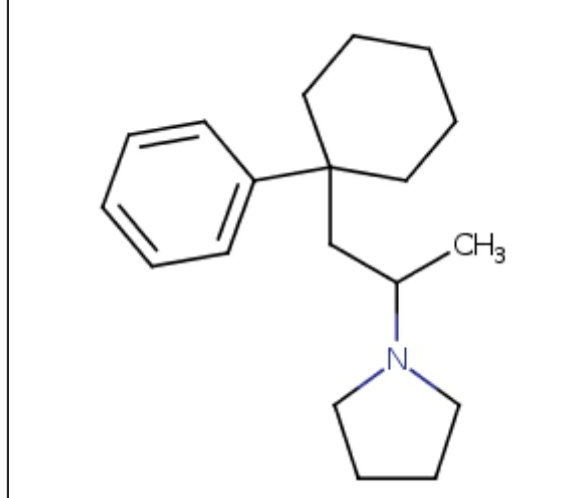
HOMO DEUS
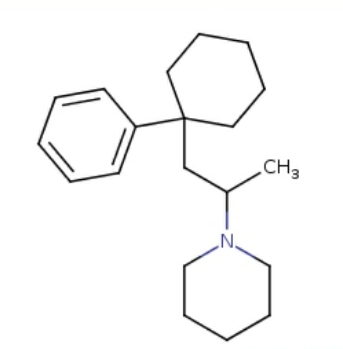
HOMO DEUS DEUS
active (very strong pbblty) at
Sigma, Sert, Dat, Vesicular Amine Transporter, D2, NET, Mu, Kappa, Delta, NMDA, adrenergic, acetylcholine, etc.
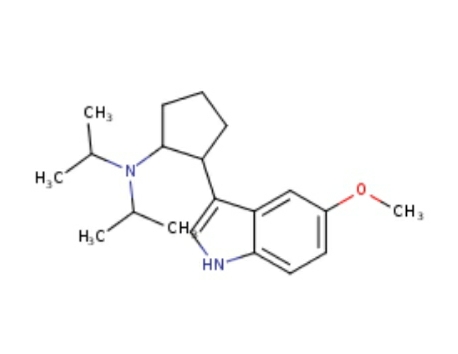
VIVENDUM
- Status
- Not open for further replies.