Sturnam
Bluelighter
- Joined
- Aug 12, 2008
- Messages
- 738
Well I'll be damned. The rumors are true. Or at least, they appear to be.
The HIV Antiretroviral Drug Efavirenz has LSD-Like Properties
Neuropsychopharmacology (2013) 38, 2373–2384; doi:10.1038/npp.2013.135; published online 3 July 2013
The HIV Antiretroviral Drug Efavirenz has LSD-Like Properties
Neuropsychopharmacology (2013) 38, 2373–2384; doi:10.1038/npp.2013.135; published online 3 July 2013
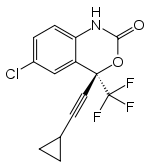
Anecdotal reports have surfaced concerning misuse of the HIV antiretroviral medication efavirenz ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluoromethyl)-2,4-dihydro-1H-3,1-benzoxazin-2-one) by HIV patients and non-infected teens who crush the pills and smoke the powder for its psychoactive effects. Molecular profiling of the receptor pharmacology of efavirenz pinpointed interactions with multiple established sites of action for other known drugs of abuse including catecholamine and indolamine transporters, and GABAA and 5-HT2A receptors. In rodents, interaction with the 5-HT2A receptor, a primary site of action of lysergic acid diethylamine (LSD), appears to dominate efavirenz’s behavioral profile. Both LSD and efavirenz reduce ambulation in a novel open-field environment. Efavirenz occasions drug-lever responding in rats discriminating LSD from saline, and this effect is abolished by selective blockade of the 5-HT2A receptor. Similar to LSD, efavirenz induces head-twitch responses in wild-type, but not in 5-HT2A-knockout, mice. Despite having GABAA-potentiating effects (like benzodiazepines and barbiturates), and interactions with dopamine transporter, serotonin transporter, and vesicular monoamine transporter 2 (like cocaine and methamphetamine), efavirenz fails to maintain responding in rats that self-administer cocaine, and it fails to produce a conditioned place preference. Although its molecular pharmacology is multifarious, efavirenz’s prevailing behavioral effect in rodents is consistent with LSD-like activity mediated via the 5-HT2A receptor. This finding correlates, in part, with the subjective experiences in humans who abuse efavirenz and with specific dose-dependent adverse neuropsychiatric events, such as hallucinations and night terrors, reported by HIV patients taking it as a medication.