AlsoTapered
Bluelighter
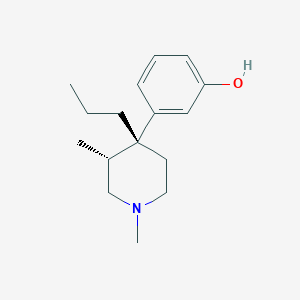
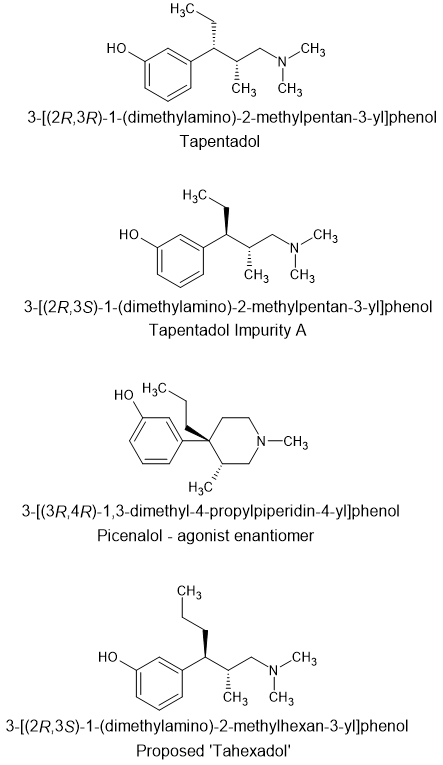
I have mentioned elsewhere that tapentadol is an open-ring analogue of picenalol.
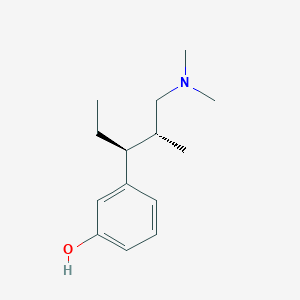
A closer look at the stereochemistry shows that the agonist enantiomer of picenalol does not overlay tapentadol but rather 'tapentadol impurity A'. It is recognised that tapentadol impurity A has significantly higher affinity for the MOR but lacked the NRI activity and thus has a significantly different analgesic profile.
 pubchem.ncbi.nlm.nih.gov
pubchem.ncbi.nlm.nih.gov
 pubchem.ncbi.nlm.nih.gov
pubchem.ncbi.nlm.nih.gov
What is more, Wyeth, Lilly and others had previously discovered that the benzylic side-chain of a meta-phenol A-aromatic demonstrates that a methyl will produce antagonist activity, an ethyl will provide mixed agonist/antagonist activity while and n-propyl will produce pure agonist activity.
So I propose 'tahexadol' i.e. 3-[(2R,3S)-1-(dimethylamino)-2-methylhexan-3-yl]phenol which as well as being a trans isomer, also has an n-propyl benzylic side-chain. I don't for one minute expect this product to be hugely potent but based on known QSAR data, would appear to suggest a potency around thatof morphine.
I would also suggest that the potency of tapentadol impurity A was known when the DEA (US) and Home Office (UK) both chose to place tapentadol into their most controlled class of drug because of previously demonstrated problems with legally defining 'stereoisomers' thus the most POTENT isomer was chosen as the baseline.
BTW it's also worth noting that like the opioid 3-(dimethylamino)-2,2-dimethyl-1-phenylpropan-1-one, the N,N-dimethyl homologue represents the only symmetrical alkyl substitution with significant activity BUT like diampromide, the N-methyl-N-(2-phenylethyl) derivative may prove to be more potent.
A closer look at the stereochemistry shows that the agonist enantiomer of picenalol does not overlay tapentadol but rather 'tapentadol impurity A'. It is recognised that tapentadol impurity A has significantly higher affinity for the MOR but lacked the NRI activity and thus has a significantly different analgesic profile.
3-[(3R,4R)-1,3-dimethyl-4-propylpiperidin-4-yl]phenol
3-[(3R,4R)-1,3-dimethyl-4-propylpiperidin-4-yl]phenol | C16H25NO | CID 13787832 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
Tapentadol impurity A
Tapentadol impurity A | C14H23NO | CID 23654757 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
What is more, Wyeth, Lilly and others had previously discovered that the benzylic side-chain of a meta-phenol A-aromatic demonstrates that a methyl will produce antagonist activity, an ethyl will provide mixed agonist/antagonist activity while and n-propyl will produce pure agonist activity.
So I propose 'tahexadol' i.e. 3-[(2R,3S)-1-(dimethylamino)-2-methylhexan-3-yl]phenol which as well as being a trans isomer, also has an n-propyl benzylic side-chain. I don't for one minute expect this product to be hugely potent but based on known QSAR data, would appear to suggest a potency around thatof morphine.
I would also suggest that the potency of tapentadol impurity A was known when the DEA (US) and Home Office (UK) both chose to place tapentadol into their most controlled class of drug because of previously demonstrated problems with legally defining 'stereoisomers' thus the most POTENT isomer was chosen as the baseline.

BTW it's also worth noting that like the opioid 3-(dimethylamino)-2,2-dimethyl-1-phenylpropan-1-one, the N,N-dimethyl homologue represents the only symmetrical alkyl substitution with significant activity BUT like diampromide, the N-methyl-N-(2-phenylethyl) derivative may prove to be more potent.




