AlsoTapered
Bluelighter
Library Genesis
Page 398, section 11.4 4-Pipedidinols in the book 'Opiate Analgesics - Chemistry and Receptors' by Casy & Parfitt
A large number of compounds were tested and although the book incorrectly identifies the MOST potent example as being:
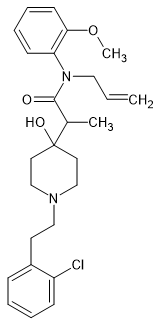
2-[1-[2-(2-chlorophenyl)ethyl]-4-hydroxypiperidin-4-yl]-N-(2-methoxyphenyl)-N-prop-2-enylpropanamide
With an ED50 of 0.07 mg/kg compared to 3.4 mg/kg for morphine in animal models i.e. around x48 morphine, it represents a facile target BUT what is actually of MORE value is the detail that both the A & B aromatics are substituted. The A-aromatic possesses an O-methoxy although the O-ethoxy was almost equipotent. The B-aromatic has an O-chloro moiety. Now the former substitution is also seen in diphenpipenol ((S) isomer is x105 morphine), an MT-45 derivative that is 118 times more potent than the parent compound.
The important question is WHY. I strongly believe it's because the o-methoxy fixes the rotation of the A-aromatic. So the question is, has someone applied this lesson to, say, fentanyl? The answer is yes they have.
Library Genesis
Compound 3 is O-methoxy fentanyl.
Is it more potent than the parent compound? Yes, 4 times as active, from the VERY limited study.
From which we can deduce that keeping the rotation of the A-aromatic relative to the nearby piperidine or piperizine (i.e. the moiety containing the basic nitrogen) is key to increasing MOR affinity.