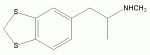
sci finder research
I just looked in the sci finder : The compound is not known, the normal phenethylamine is also not known. The Thioanalogues of safrol or isosafrol is also unknown, but the aldehyde is mentioned twice:
Preparation of 1,3-benzoxathiole derivatives for treatment of liver disease. Ueyama, Tamio; Matsumoto, Kazuo; Hirase, Joji; Oosugi, Yumi; Ooshima, Hiroichi; Sogawa, Takeshi; Mizuno, Yasuo; Okui, Yoshikazu; Nishino, Minoru; Nishigaito, Toshinori. (Shinnippon Yakuhin Kk, Japan). Jpn. Kokai Tokkyo Koho (1994), 40 pp. CODEN: JKXXAF JP 06293640 A2 19941021 Heisei. Patent written in Japanese. Application: JP 94-18503 19940215. Priority: JP 93-25199 19930215. CAN 123:169595 AN 1995:780252 CAPLUS
Patent Family Information
Patent No. Kind Date Application No. Date
JP 06293640 A2 19941021 JP 1994-18503 19940215
Priority Application
JP 1994-18503 A 19940215
JP 1993-25199 19930215
Abstract
Title compds. I (R = H, F, Cl, cyano, alkyl, alkoxy, amino; n = 1, 2; X = O, S; Y = CS, CH2) and their pharmacol. acceptable salts, useful for treatment of liver disease, were prepd. Thus, stirring 2-mercapto-4-(methylthio)phenol with thiophosgene and aq. NaOH in CHCl3 for 30 min gave 5-methylthio-1,3-benzoxathiole-2-thione (II). II showed 46% inhibition of D-galactosamine-induced hepatic injury at 12.5 mg/kg orally in rats. Formulations contg. I were given.
and
Stabilizing solution for silver halide photographic material and photographic processing using same. Tsuchiya, Ichiro; Haraguchi, Takeshi; Koboshi, Shigeharu. (Konica Co., Japan). Jpn. Kokai Tokkyo Koho (1992), 40 pp. CODEN: JKXXAF JP 04311957 A2 19921104 Heisei. Patent written in Japanese. Application: JP 91-104699 19910410. Priority: . CAN 118:157730 AN 1993:157730 CAPLUS
Patent Family Information
Patent No. Kind Date Application No. Date
JP 04311957 A2 19921104 JP 1991-104699 19910410
Priority Application
JP 1991-104699 19910410
Abstract
The title stabilizing soln. contains one or more benzodioxole derivs., or analogs thereof (Markush structure given). The surface tension of the title material is 15 to 60 dyne/cm at 20°. Compd. I is an example of the said benzodioxole derivs. The title soln. has good storage stability. Also claimed is the title processing method.
So go on guys and try it out!