Transform
Bluelight Crew
- Joined
- Sep 5, 2010
- Messages
- 4,808
Anyone care to speculate about the possibility for CTMP to be dangerously hydroxlyated? I expect the ester will be easily hydroylsed but anecdotal reports suggest a long active half life, so dichloro ritalinic acid must be active too. Could this be dangerous?
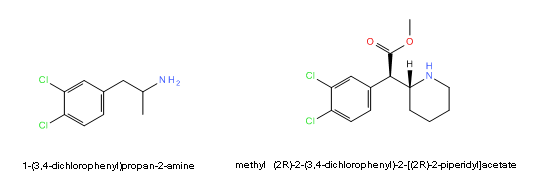
Dichloroamphetamine < < < < > > > > 3,4-CTMP

Dichloroamphetamine < < < < > > > > 3,4-CTMP
Now read this from the Rhodium archive, and bear with me, it won't all be technical jibber jabber and you may want to hear my actual argument:This suggests that given enough room, some hydroxylase apparently oxidizes the halogenated position transfering the Cl to the adjacent position on the phenyl (called NIH shift as mentioned).An unusual aspect of 4-CA metabolism is the reported conversion of the drug to oxygen-containing products. A phenolic product was identified by Parli and Schmidt (1975) as being 3-chloro-4-hydroxyphenylisopropylamine. This would seem to invoke the NIH shift as an explanation for the migration of the chloro atom. Even more remarkable is the report (Sherman and Gal, 1976) of the isolation of 3,4-dimethoxyphenylisopropylamine following the intraventricular injection of 4-CA. This represents the formation in vivo of a weak but accepted pressor and psychotomimetic. When the mechanism of its formation is understood, a chemical link may be at hand tying the simpler phenylisopropylamine stimulants to the methoxylated psychotomimetics. There were no reports from the clinical studies of 4-CA that suggested any psychotomimetic action.
In such a compound as DOC or 2C-C there may be too much steric hindrance or the different electronic distribution making it too hard for such an enzyme to gobble on the Chloro.
However if 3,4-dichloroamphetamine also shows toxicity we may want to suspect something similar happening since the 4-chloro can still be relocated to the 5-position and/or the 3-chloro to the 2-position.
I must admit I was skeptic at first of polymath concerns, focussing on the various structural differences rather than the commonalities, but I think the above suggests that we should avoid a compound like this because of the likelihood of shady metabolites by hydroxylase.
Because you really don't want phenolic metabolites, I seem to recall F&B also avoiding anything that might lead to that like the plague and rightly so.
Also I think one of the most similar compounds to this CTMP is dichloropane, I am surprised no one has mentioned it and am also curious if that compound was tested for Raphe nuclei dopaminergic toxicity and things like that.
Then there is also AH-7921 with the same dichlorophenyl pattern but that might be too far from dopaminergic to be relevant. Still if you are a user of that or considering it, this could deter you.
Anyway I saw this drug (CTMP) being offered and after having the thoughts I just expressed decided that it would almost be hubristic to proceed trying it. (Apart from the fact that stimulants have pretty much disappeared from my diet)
If I haven't made myself very clear, ask.



