paracelsius
Bluelighter
- Joined
- Mar 11, 2020
- Messages
- 197
just trying to get my head around that one: why is this opioid specifically excluded from Schedule in Canada.??
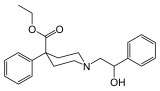
I mean this compound is a potent mu opioid agonist about 5x morph, ~10x more potent than pethidine. but yet morph, and pretty much all piperidines are classified but not that specific opioid. Technically that means it is perfectly legal in Canada or am i missing something?

lack of addictive potential?? hmmm not sure how the UN functionaries come up with that conclusion!!Oxpheneridine[1] (Carbamethidine) is a 4-phenylpiperidine derivative that is related to the opioid analgesic drug pethidine (meperidine)....In Canada, Oxpheneridine is specifically excluded from the illegal drugs list on the Controlled Drugs and Substances Act schedules, presumably on the basis of the lack of addictive potential found by the UNODC (wiki)
I mean this compound is a potent mu opioid agonist about 5x morph, ~10x more potent than pethidine. but yet morph, and pretty much all piperidines are classified but not that specific opioid. Technically that means it is perfectly legal in Canada or am i missing something?

