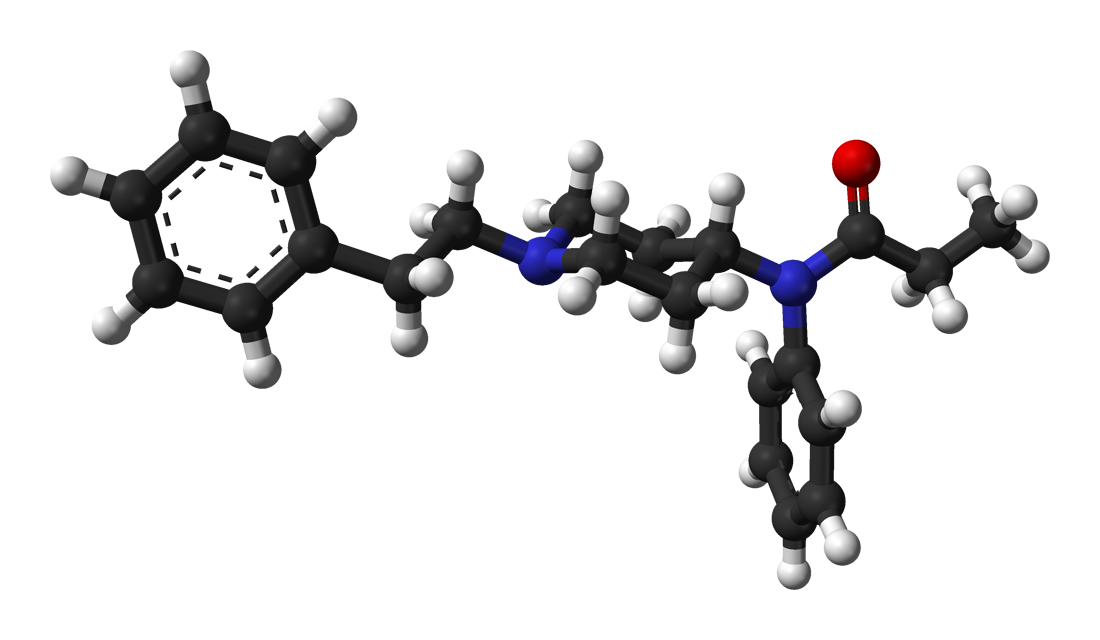
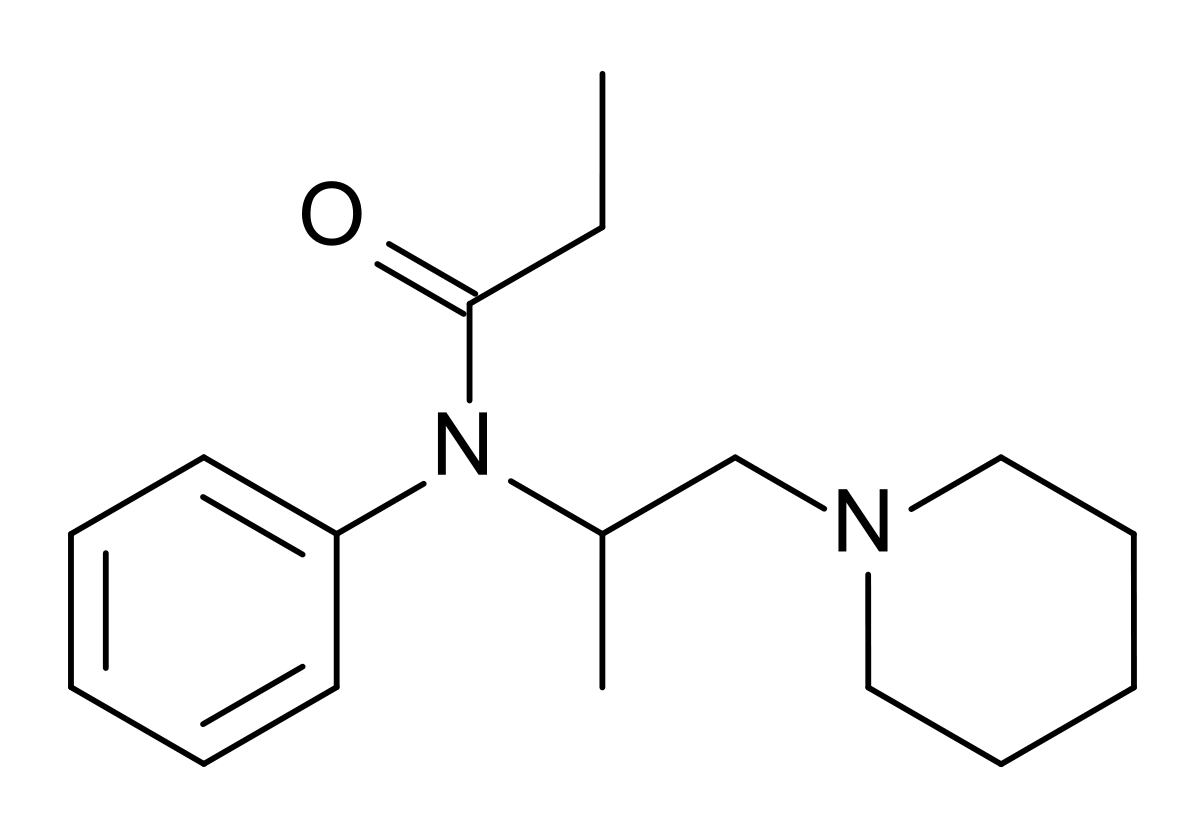
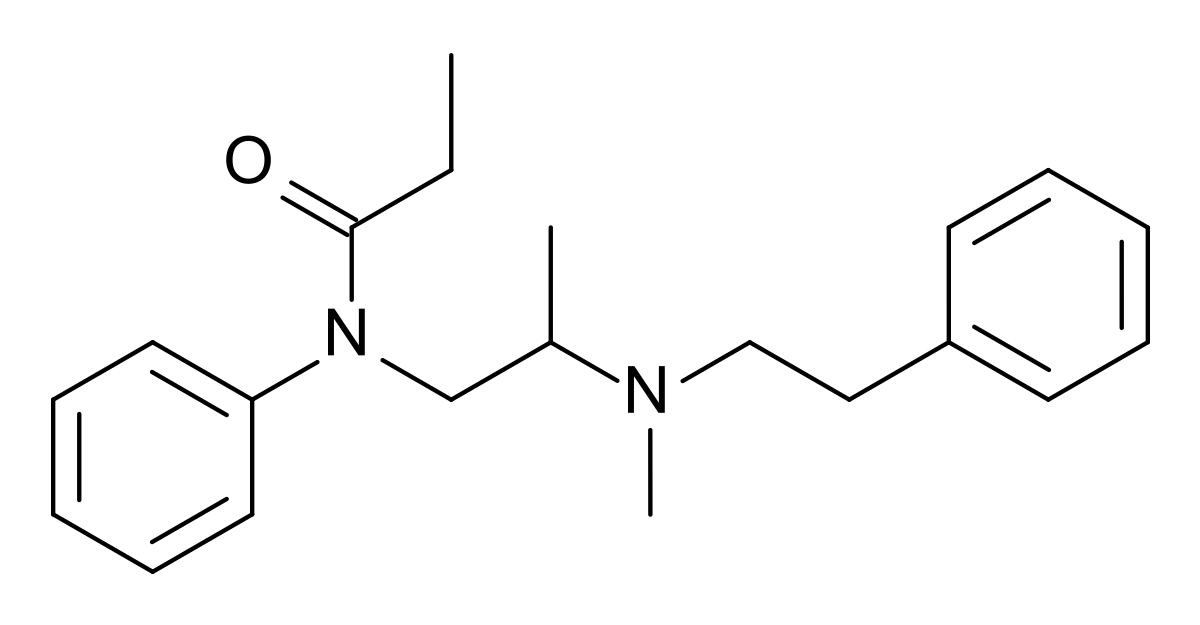
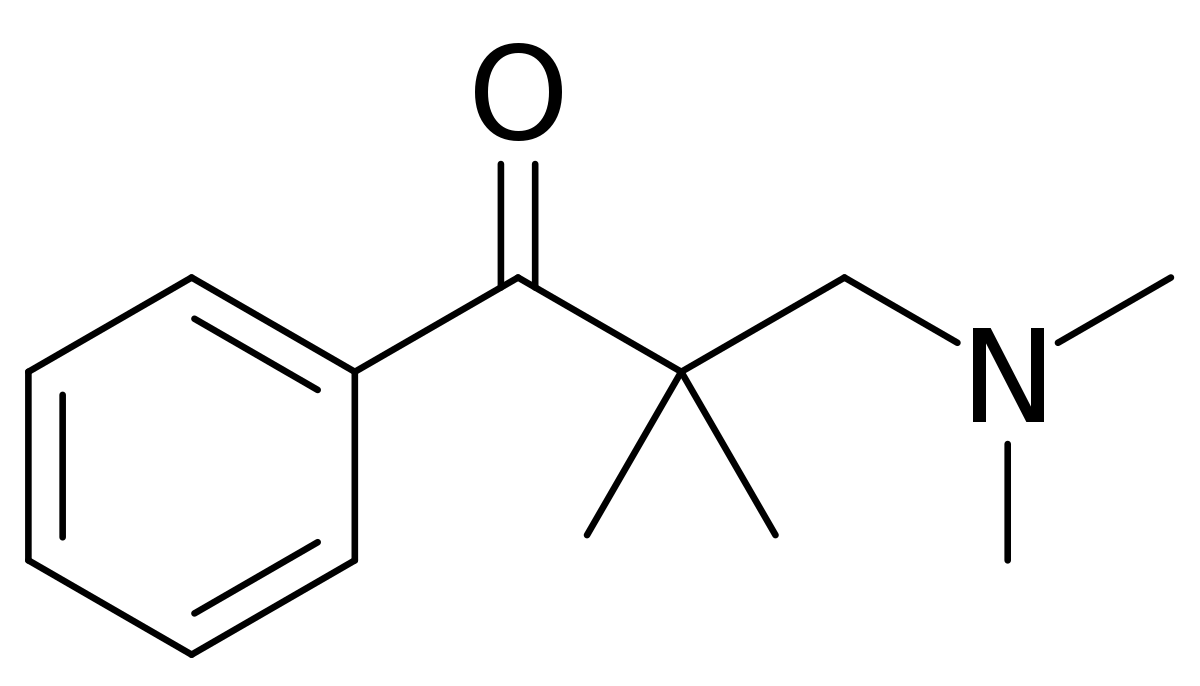
Unfortunately the therapeutic index
looks pretty bad.
I would also be careful with nitro groups in drugs that haven't gone through clinical trials, they are
often associated with toxicity and tend to be avoided in modern drug discovery programs unless there's a good reason to keep it.
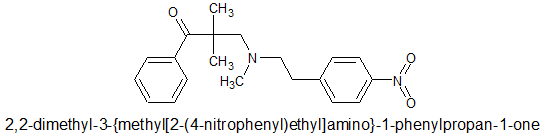
I have very closely considered the metabolic pathways for any compound I suggest. The nitro group is found in several other opioids that HAVE been through trials, specifically as p-nitro phenylethyl moieties. as I pointed out. In all cases the result is reduction (unusual since the body is generally an oxidative instrument) Aromatic amines most certainly can be mutagenic but only because they are on an aromatic with further substitutions and said heterocycles are mutagenic and/or affect hERG proliferation. N-dealkylation & hydroxylation of the dimethyl moieties are the other pathways. A small amount of p-hydroxylation of the benzene ring will also be expected.
Please don't think I'm not appreciative of people sounding notes of caution but I have quite a lot of experience in drug design. I am prepared to try things I design but if it looks good, it goes to Huntingdon Life Sciences for animal models. I was first into man so my wife is then first into woman, my son was first into son. That was my gauge, I would ask 'would I be happy for Ollie to take this' and I threw some away. Some that later turned out to be dangerous. Methoxyphenidine, for example.... the bk-2Cx series. The former was toxic, the latter could dimerize and the metabolic pathway could get too complex to work out.
Like all things, it's 1% inspiration, 99% inspiration. I didn't just come up with the compound today, it's something I have been considering for about 2 years. I wanted to collect all of the related patents & related compounds & then to finding ALL of the metabolic pathways.
Oh, I've just remembered that in example 11 of US Patent 3719669 (See Wiki aniliopam), they also disclose the p-Nitro-2-phenylethyl analogue. The reason the p-Nitro-2-phenylethyl analogues aren't seen much is because their duration is short (40 minutes or so) before they are reduced to the p-amino-2-phenylethyl which us generally about ¼ the potency of the nitro. As you can imagine, they do not find much medical application because their would be a 2-phase metabolism. You would not be able to keep giving the p-Nitro without the p-Amino accumulating (lasts 2-3 hours(.... so the p-aminos are more usually seen.
I have just realised that tapentadol is a mixed agonist antagonist and to make a FULL agonist, all you need is 1 extra methyl group thus:
Image Tahexadol hosted in ImgBB

ibb.co
Tahexadol anyone?
Oh, an anybody wondering why making the side-chain 1 carbon longer will produce a full agonist, look at the patents on (3R,4R) Picenadol.... or just convert them to 3D, calculate the minimum-energy state & overlay them. It's also worth considering JDTic. A phenolic opioid with an alkyl side-chain on the alpha carbon follows a simple rule. Methyl = antagonist, Ethyl = mixed agonist/antagonist Propyl = agonist.
The thing is, the patent on tapentadol NEVER mention an example of an N-propyl side-chain. It's like the butorphanol patents that only provide examples that are mixed agonist/antagonist or pure antagonists BUT luckily, a research team from the 1950s tried every possible N-substitution:
https://www.unodc.org/unodc/en/data-and-analysis/bulletin/bulletin_1958-01-01_4_page007.html