As I see that there aren't many (none?) threads about blue lilly/lotus, and considering that my experience is STILL limited with it, I've decided to put in this thread my experience with the plant, or well, with Nuciferine alkaloids and Aporphine, as I have used both Nelumbo Nucifera and Nymphaea Ceruleae.
My most interesting experience was when I mixed Damiana with Klip Dagga and Nelumbo resin. It was quite similar to cannabis but also quite different, more "static" and dreamy, but less euphoric or psychedelic.
Before this I had consumed poor quality flowers of nymphaea ceruleae in tea...
Very disappointing. I spoke to
@Kaleida about this and she told me that maybe the kratom could have messed with Nymphaea's high, as Nymphaea's high is very subtle and some actives of kratom hit the same receptors (D2).
Other experiments smoking Nelumbo stamens, Nelumbo resin with other plants or Nymphaea flowers were also quite disappointing...
My last attempt has been to put many grams (15? ) of Nymphaea plus 10-15g of Nelumbo stamens in a mason jar and cover it with red wine, of two different types, oh well, that's what was there at the time...
I have left the wine in the jug for more than a month, I have stirred it, but not every day. The result is a wine that is somewhat redder and denser than normal, with a somewhat stronger smell and a rather vinegary taste, probably because I have left it for too long and have not refrigerated it. In this sense it has been a mistake, but it could be drunk without much trouble. It didn't taste bitter like I've seen elsewhere.
The effects may have been due to the alcohol, but in my opinion they were somewhat different, and not the alcohol, as one of my Nymphaea teas (the one that produced some effects) had similar effects, although less intense and less interesting.
Normally the wine leaves a feeling of fast, warm and not clear-headed euphoria. In this case, the euphoria was less, but more all-encompassing, longer lasting (2-3 hours), more opioid-like and clear-headed, and with a headspace similar to that of benzodiazepines with a small degree of enhancement similar to that of very little. marijuana (sativa).
There was a very interesting feeling of peace, there were also some moments of high eroticism, I got horny for at least 30-40 minutes, and it would have been a very interesting high to do something as a couple...
The experience was too dreamy and the feeling of peace too "silent" to be placebo or simply alcohol. To tell the truth, it hardly seemed to me like a couple of glasses of wine, which would have left me in a very different mental and physical state. Perhaps the vinegar of the wine made the alcohol disappear (and also some actives'??), I have no idea.
It was an interesting experience, I'll try to do better another time, with a better source and with more care to avoid vinegar-ism, maybe leaving it for 3 weeks instead of 5-6 and refrigerated... I'll invite some interested/ing girl to Egyptian wine

:hypno:
I am now 7 days without kratom and I think that has affected the experience quite positively.

 en.wikipedia.org
en.wikipedia.org
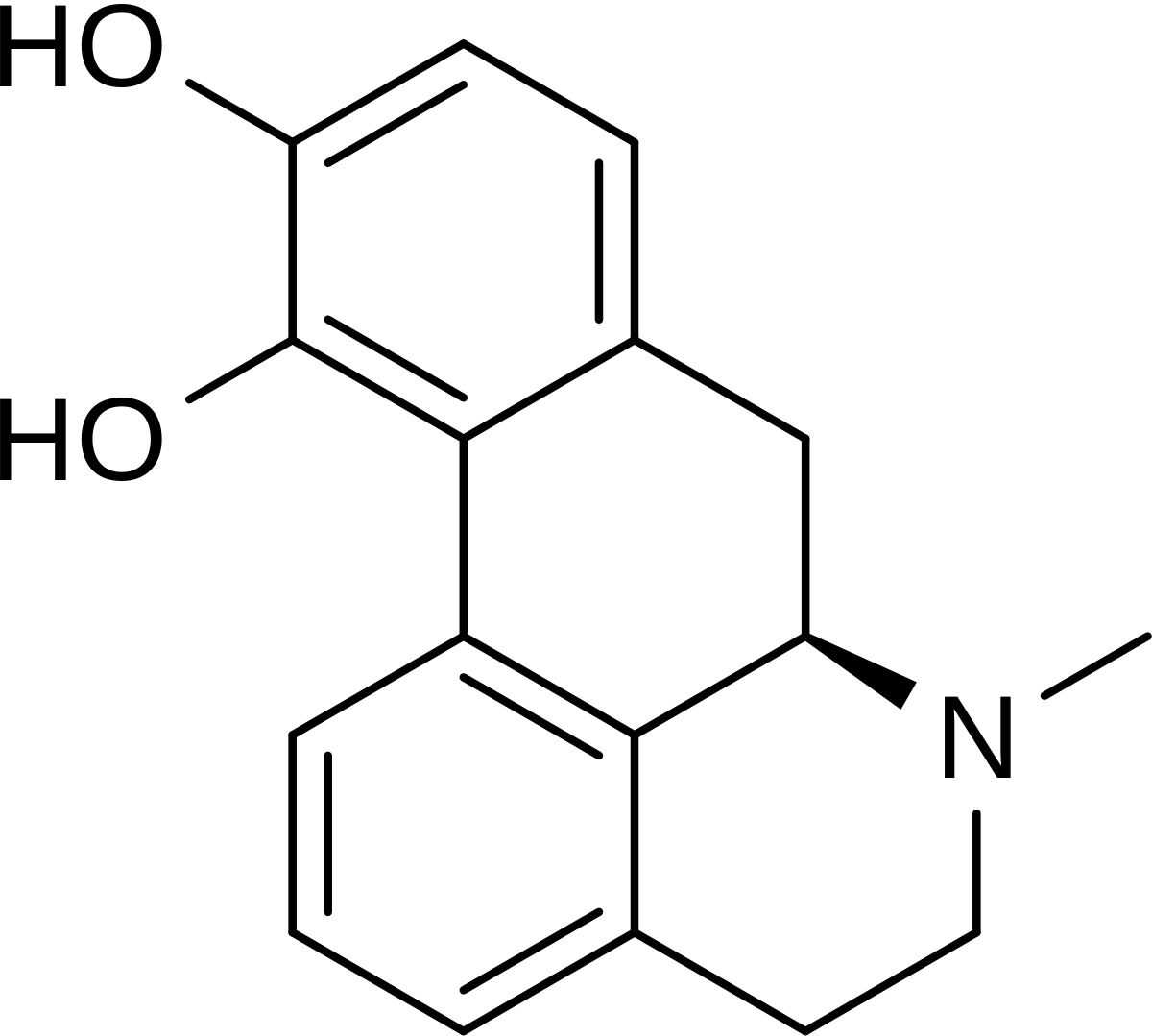
 en.wikipedia.org
en.wikipedia.org
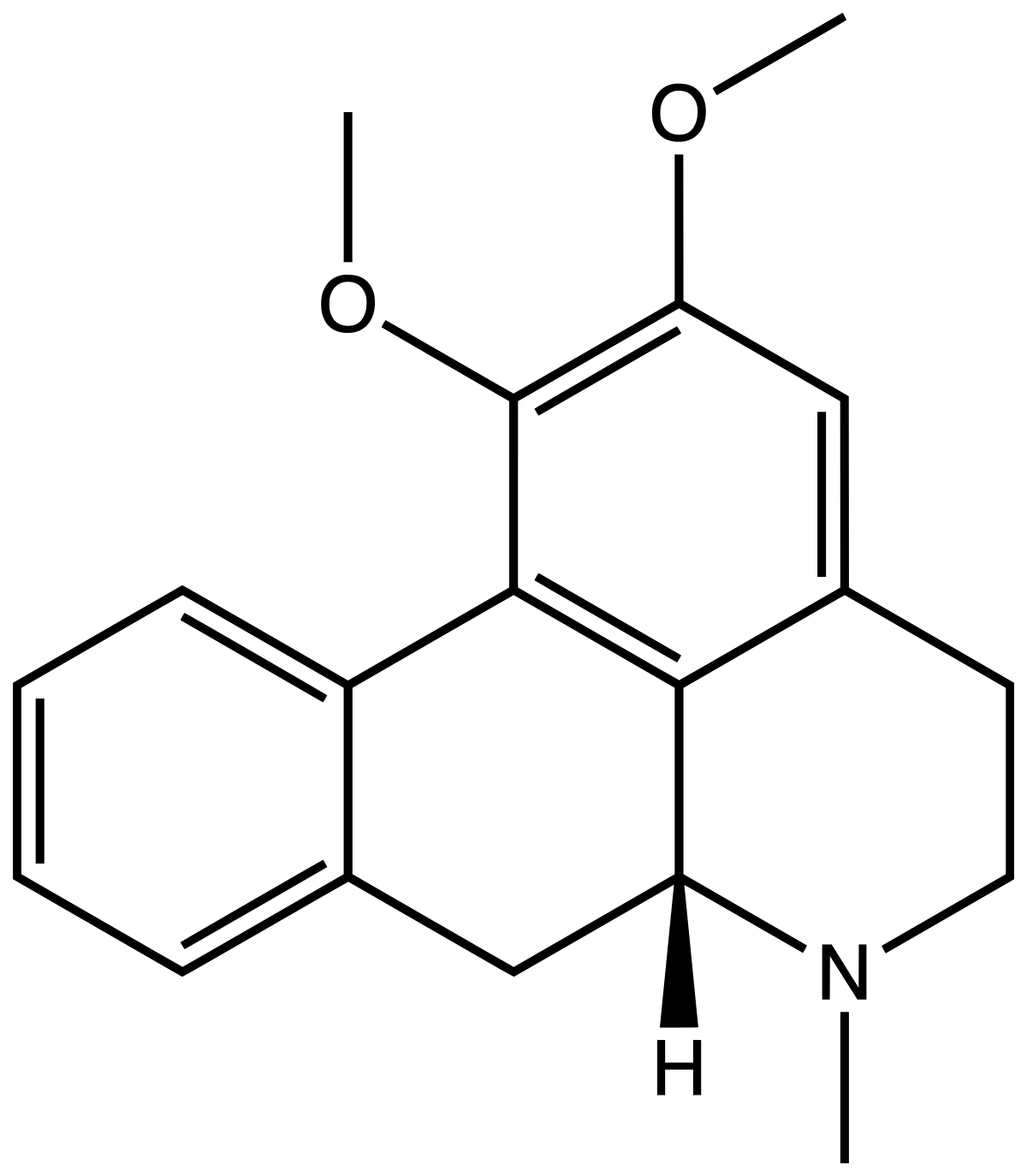
 en.wikipedia.org
en.wikipedia.org






 :hypno:
:hypno: