These are questions for our chemists. It appears to my untrained and ignorant eye that the only difference between Tryptamine and NN DMT is the radical NH2 versus N- - , as show in the attached structures. (Notice that psilocin, NN dmt and 4 aco dmt, all very active, have N - - .) Why is one (NN DMT) psychedelically active and the other, Tryptamine not? -- this is the only visible difference. Is the NH2 sufficient difference to stop activity? #2 - Then looking at Norbaeocystin: it is very similar to psilocybin but it has the same NH2 instead of N- -. Does this stop its psychactivity? I am hoping someone can shed some light on this for me, blue or otherwise (bad joke).
What is the fundamental psychoactive structure, versus those that are not active? Again Tryptamine and NN DMT are so similar, yet one is and the other not.
Does anyone have an experience with Norbaeocystin (NBC)? It looks very similar to Psilocybin (4 PO), even more than 4 aco dmt, which experientially feels and works just like psilocybin to me. One might think that NBC would be as good as 4 PO -- except that it has the NH2 as does Tryptamine which might cancel its psychoactive effect.
Why does NN DMT orally requiere a MAOI while Psilocybin, Psilocin, and 4 aco, not?
Does anyone have experience taking 5 MEO DMT orally with a MAOI?
Link to image:
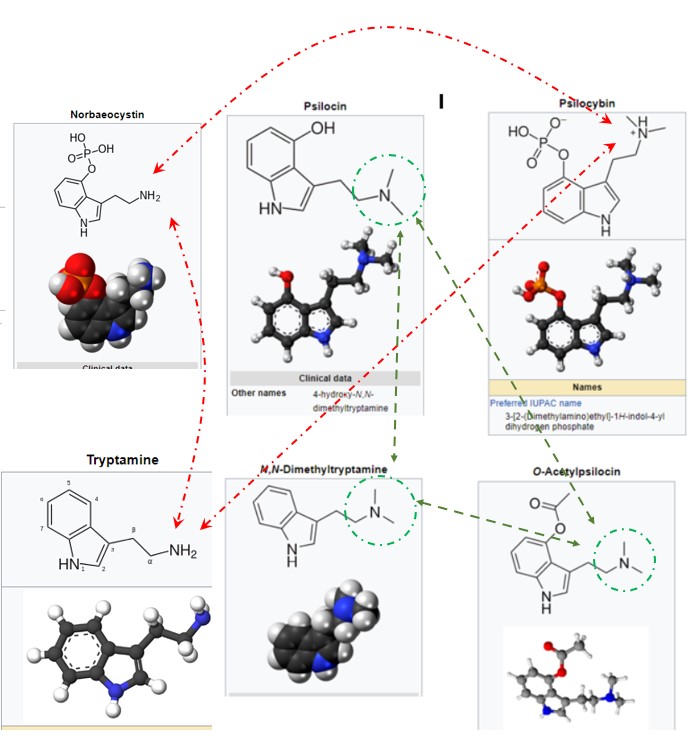
What is the fundamental psychoactive structure, versus those that are not active? Again Tryptamine and NN DMT are so similar, yet one is and the other not.
Does anyone have an experience with Norbaeocystin (NBC)? It looks very similar to Psilocybin (4 PO), even more than 4 aco dmt, which experientially feels and works just like psilocybin to me. One might think that NBC would be as good as 4 PO -- except that it has the NH2 as does Tryptamine which might cancel its psychoactive effect.
Why does NN DMT orally requiere a MAOI while Psilocybin, Psilocin, and 4 aco, not?
Does anyone have experience taking 5 MEO DMT orally with a MAOI?
Link to image:

Last edited:





