Morninggloryseed
Bluelight Crew
So I saw a picture of a crystal of bismuth (ingredient in pepto bismol) and noted how awsome they looked.

And then I thought it would be neat to incooperate that into a psychedelic. Sure enough I looked at the periodic table, and noted that nitrogen and phosphorus were in the same row. Both elements found in friendly, neighborhood psychedelics. So I thought about making a psychedelic with bismuth, because wouldn't it be awsome to trip on something so pretty!
Then just today, I was reading about 2C-T-13 and read Shulgin say....
Low and behold, phosphorus and nitrogen are in the same row as bismuth! So I am thinking, this may actually work.
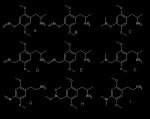
Here are a few obvious bismuth analogues I came up with.....

Personally, I'd put my money on D and G being active. But you guys who have real knowledge of pharmocology, neuropharmocology, and chemistry...what do you think of the prospects of these?
And then I thought it would be neat to incooperate that into a psychedelic. Sure enough I looked at the periodic table, and noted that nitrogen and phosphorus were in the same row. Both elements found in friendly, neighborhood psychedelics. So I thought about making a psychedelic with bismuth, because wouldn't it be awsome to trip on something so pretty!
Then just today, I was reading about 2C-T-13 and read Shulgin say....
Most of the synthetic adventures of putting a basic something aways out from the benzene ring, at the four-position, have involved subtle things such as unsaturated bonds or three-membered rings. This was the first try with the actual use of a different atom (an oxygen). What about other heteroatoms such as sulfur or nitrogen or silicon or phosphorus, or some-such?
Low and behold, phosphorus and nitrogen are in the same row as bismuth! So I am thinking, this may actually work.
Here are a few obvious bismuth analogues I came up with.....
Personally, I'd put my money on D and G being active. But you guys who have real knowledge of pharmocology, neuropharmocology, and chemistry...what do you think of the prospects of these?