MedicinalUser247
Music Ambassador
- Joined
- Aug 2, 2023
- Messages
- 4,746
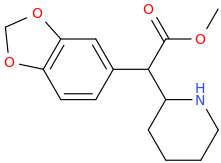
I'm just posting to post about MDMA compared 3,4 Methylene Dioxy MethylMethylphenidate. Which one do you think is more euphoric ? Although 3,4 Methylene Dioxy MethylMethylphenidate would be more euphoric hypothetically. There still haven't been too many comparisons between the two molecules. So, I want to get your opinion on it. So, which one do you think is stronger MDMA or 3,4 Methylene Dioxy MethylMethylphenidate ?