djsim
Bluelight Crew
- Joined
- Mar 18, 2007
- Messages
- 3,220
Hey all, I know his is highly hypothetical... but here goes.
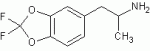
Anyway, I just finished doing a review on MDMA for one of my uni classes. I had to draw up a bunch thing like MDMA, MDA, MMDA (MDMA with methoxy at 5 position)... anyway, I fell asleep while drawing and kinda dreamed about what i was doing when i fell asleep.
Is this compound possible?
[attachment]
Curiosity just got the better of me. I know it would be very hard to find a precursor with those substitutions on the benzene, but yeh, is it possible? Would you expect it to have activity? The strain would be pretty great though, yeh?
Your thoughts appreciated
Anyway, I just finished doing a review on MDMA for one of my uni classes. I had to draw up a bunch thing like MDMA, MDA, MMDA (MDMA with methoxy at 5 position)... anyway, I fell asleep while drawing and kinda dreamed about what i was doing when i fell asleep.
Is this compound possible?
[attachment]
Curiosity just got the better of me. I know it would be very hard to find a precursor with those substitutions on the benzene, but yeh, is it possible? Would you expect it to have activity? The strain would be pretty great though, yeh?
Your thoughts appreciated