Fertile
Bluelighter
- Joined
- Mar 31, 2022
- Messages
- 1,627
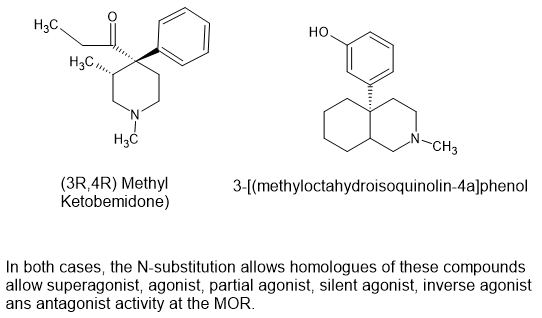
A few years ago I looked into ketobemidone derivatives. I found NONE. I was somewhat surprised but I noticed 2 things. Firstly, none of the studies resolved the stereoisomers and we know from other opioids which display mixed agonist/antagonist properties BECAUSE the product is a mixture of 2 isomers - Viminal (Dividol) being a prime example.
Then I discovered a compound closely related to ketobemidone, namely picenadol (LY-97435). The (3R,4R) isomer is an agonist while the (3S,4S) isomer is an antagonist.
It seems likely that the reason is that ketobemidone was developed by Otto Eisleb at Winthrop Chemical Corporation in 1938 when stereoisomerism of new drugs went unexplored.
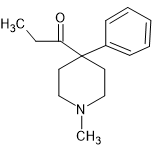
Eisleb tried various different N-substitution patterns (more than a dozen) and whatever he did, the result was an antagonist. Pages 332-333 of 'Opiates' (Lenzm Evans, Walters & Hopfinger) details the many substitutions that were tested and while the N-methyl has agonist activity, while more potent analgesics were found, they conferred mixed activity.
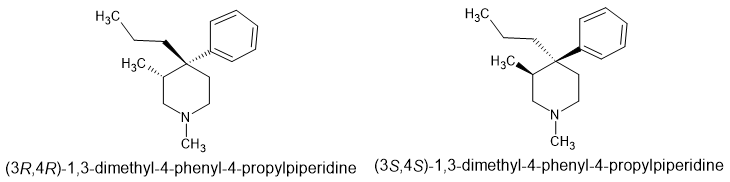
But by 1975, Dennis M. Zimmerman at Eli Lilly revisited the ketobemidone scaffold and using newly discovered information, the 4 ethyl ketone was replaced by a simple N-propyl at the 4 position and a 3-methyl was introduced. Stereoisomerism WAS tested and it was found that while one isomer was an agonist, the other was an antagonist so even if Eisleb had introduced such a 3-methyl, the product would show no effect when tested on animal models.
But what is of most interest is that IF the agonist stereoisomer IS a pure agonist, will N-substituents normally associated with potent agonist activity be compatible. Eisleb tried these substitutions - substitutions that provide high potency when applied to prodine (the compound closest to 3-methyl ketobemidone)..

H should add that it's likely that the (3S,4S) stereoisomers is likely to be an agonist, but much less potent. So in practice the key is to remove just 1 stereoisomer.
I do not see this as a practical target, but it is of value when understanding the stereoisomers as they apply to phenolic opioids.
Then I discovered a compound closely related to ketobemidone, namely picenadol (LY-97435). The (3R,4R) isomer is an agonist while the (3S,4S) isomer is an antagonist.

It seems likely that the reason is that ketobemidone was developed by Otto Eisleb at Winthrop Chemical Corporation in 1938 when stereoisomerism of new drugs went unexplored.

Eisleb tried various different N-substitution patterns (more than a dozen) and whatever he did, the result was an antagonist. Pages 332-333 of 'Opiates' (Lenzm Evans, Walters & Hopfinger) details the many substitutions that were tested and while the N-methyl has agonist activity, while more potent analgesics were found, they conferred mixed activity.
But by 1975, Dennis M. Zimmerman at Eli Lilly revisited the ketobemidone scaffold and using newly discovered information, the 4 ethyl ketone was replaced by a simple N-propyl at the 4 position and a 3-methyl was introduced. Stereoisomerism WAS tested and it was found that while one isomer was an agonist, the other was an antagonist so even if Eisleb had introduced such a 3-methyl, the product would show no effect when tested on animal models.
But what is of most interest is that IF the agonist stereoisomer IS a pure agonist, will N-substituents normally associated with potent agonist activity be compatible. Eisleb tried these substitutions - substitutions that provide high potency when applied to prodine (the compound closest to 3-methyl ketobemidone)..

H should add that it's likely that the (3S,4S) stereoisomers is likely to be an agonist, but much less potent. So in practice the key is to remove just 1 stereoisomer.
I do not see this as a practical target, but it is of value when understanding the stereoisomers as they apply to phenolic opioids.