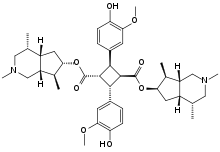
Abstract: Incarvillateine (1), a new monoterpene alkaloid car-
rying a characteristic cyclobutane ring, has been found to show
significant antinociceptive activity in a formalin-induced pain
model in mice. To investigate the correlation between its struc-
ture and antinociceptive activity, and especially to study
whether a cyclobutane ring is necessary or not for expression
of activity, we evaluated the antinociceptive activity of two
constructive units of incarvillateine, such as a monoterpene
unit (incarvilline, 3) and a phenylpropanoid unit (ferulic acid, 2)
in the formalin test, and compared activity of the units with
that of incarvillateine. Furthermore, in order to obtain more in-
formation about the structure-activity relationships, monoter-
pene alkaloid derivatives, such as incarvine C (5, a precursor of
incarvillateine), incarvine A (4, an ester compound comprised
of two monoterpene alkaloids and a monoterpene) and 3,3¢-de-
methoxy-4,4¢-dehydroxyincarvillateine (6, a synthetic new
compound), were examined. The antinociceptive effect of 3,3¢-
demethoxy-4,4¢-dehydroxyincarvillateine was equal to that of
incarvillateine. Meanwhile, the other compounds exhibited no
or weak activity. These results suggested that the cyclobutane
moiety of incarvillateine plays an important role in expression
of antinociceptive action.

 en.wikipedia.org
en.wikipedia.org

 en.wikipedia.org
en.wikipedia.org







