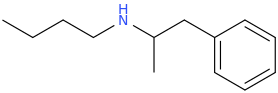
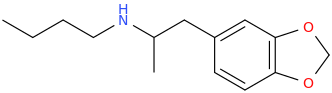
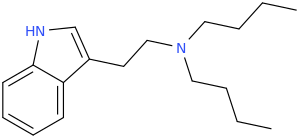
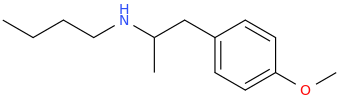
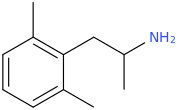
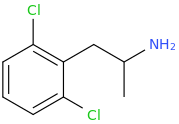
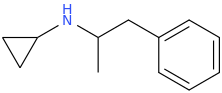
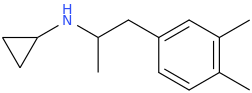
-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Ketamine salts solubility
- Thread starter fastandbulbous
- Start date
- Status
- Not open for further replies.
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
I think Dave Nichols beat you to it, by several years.I think it would be intresting to see if someone could come up with Lysergic Acid 2,4 Dimethylaziridine. Hypothetically it could be stronger than LSD.
It would help if you put the ends of the bonds where they're supposed to be and not in the middle of nowhere? If you ask nicely, I'll send you a copy of ChemDraw.Well... I came up with my own LSD design. What do you think of this ? https://imageshack.com/i/pnhiPTiLp
AlsoTapered
Bluelighter
- Joined
- Apr 1, 2023
- Messages
- 3,076
It would help if you put the ends of the bonds where they're supposed to be and not in the middle of nowhere? If you ask nicely, I'll send you a copy of ChemDraw.
Chem3D was the element I used the most... but I guess if someone presumes PEAs are flat, ChemDraw is sufficient. The only annoying thing I found was that if you made the minimum-energy conformation calculations too granular, Chem3D could chug away for a fair time... but obviously not with such low MW compounds.
MedicinalUser247
Bluelighter
- Joined
- Aug 2, 2023
- Messages
- 911
If ChemDraw works on my computer. I'd Thank you very much for giving it to me. As far as what I was going to post about. Do you think LSD could be crossed with a Tryptamine like this ? Or is this just wishful thinking ? https://imageshack.com/i/pmWIxopOp
- Joined
- May 11, 2011
- Messages
- 3,392
My guess would be that the bulk there would abolish activity. LSD is pretty optimized for the serotonin 2A receptor, hence there not being too many changes that gain or even preserve activity.If ChemDraw works on my computer. I'd Thank you very much for giving it to me. As far as what I was going to post about. Do you think LSD could be crossed with a Tryptamine like this ? Or is this just wishful thinking ? https://imageshack.com/i/pmWIxopOp
- Joined
- May 11, 2011
- Messages
- 3,392
The bulk where the pentyl chain of THC would be would decrease activity. The active cannabinoid in Radula marginata, perrottetinene has a phenethyl group, which aproximates the length of the pentyl in THC, but is a partial agonist. Adding even more bulk would be inactive at CB receptors (especially with that dimethylamine at the end. That would totally ruin the hydrophobic interactions that the CB receptors require. Heres a couple pictures of my designs. The first is THC crossed with a tryptamine I call it tetryptamine. The second is a molecule I call MDA1. https://imageshack.com/i/pmzapGuXp https://imageshack.com/i/pnvD6N0Ap
Heres a couple pictures of my designs. The first is THC crossed with a tryptamine I call it tetryptamine. The second is a molecule I call MDA1. https://imageshack.com/i/pmzapGuXp https://imageshack.com/i/pnvD6N0Ap
Unfortunately all of the bulk of the THC portion of the molecule would likely abolish 5HT2A binding. DMT itself can't take much more than a methoxy at the 5 position, and 6 position modifications seem to abolish activity of tryptamines.
Triptans are the tryptamines with the most bulk on the phenyl ring. They have some serotonergic activity as antimigrane drugs, but are not psychadelic. The prototypical triptan, sumatriptan, is 5-N-methyl-methanesulfonamide-DMT, and that substituient is much smaller than the THC portion you have suggested. It also is highly charged and an H-bonder, so it is even more likely that molecule would be inactive.
Your second compound actually exists, as it was made by Shulgin in PIHKAL (it was kind of a side note in the MDA entry). It is called Alpha, and has some mild dreamyness, with no anorexia at any dose tried but not any other notes on it. Seems interesting.
Just a question @MedicinalUser247, but what is your background in drugs/chemistry that makes you interested in this kind of research?
MedicinalUser247
Bluelighter
- Joined
- Aug 2, 2023
- Messages
- 911
Well... earlier in my life I wanted to become a doctor or pharmacologist, but those dreams were cut short when I dropped out of high school. Later on in my life I wanted to get my GED and than go into the medical field, but that never happened because I kept dropping out of my GED classes due to my Schizophenia. So, I guess you can say pharmacology is just a hobby now and I feel too old to go back to school and study.
- Joined
- May 11, 2011
- Messages
- 3,392
That's cool. If you ever want some resources to self study, I can help you find something.Well... earlier in my life I wanted to become a doctor or pharmacologist, but those dreams were cut short when I dropped out of high school. Later on in my life I wanted to get my GED and than go into the medical field, but that never happened because I kept dropping out of my GED classes due to my Schizophenia. So, I guess you can say pharmacology is just a hobby now and I feel too old to go back to school and study.
AlsoTapered
Bluelighter
- Joined
- Apr 1, 2023
- Messages
- 3,076
That's cool. If you ever want some resources to self study, I can help you find something.
We have posted all manner of useful resources on this thread. But you know how it is with things like medicinal chemistry - 1% inspiration, 99% perspiration. The Useful Links thread has several books and several hundred hot-links to articles that I've added and that's just a fraction of what's there, free. I've done all the heavy-lifting to avoid duplication of effort.
- Joined
- May 11, 2011
- Messages
- 3,392
A lot of your resources are pretty high level. I was more thinking like uni level organic chemistry stuff like MIT's open coursewear offering for organic chemistry I. That would allow for Medicinal User to better utilize the wealth of resources that you have provided on the medicinal chemistry front.We have posted all manner of useful resources on this thread. But you know how it is with things like medicinal chemistry - 1% inspiration, 99% perspiration. The Useful Links thread has several books and several hundred hot-links to articles that I've added and that's just a fraction of what's there, free. I've done all the heavy-lifting to avoid duplication of effort.
Ambrisentan
Greenlighter
- Joined
- Jun 28, 2021
- Messages
- 16
phenidatazine
- Status
- Not open for further replies.