AlsoTapered
Bluelighter
Espacenet – search results
Espacenet: free access to millions of patent documents. Find out if your invention is unique or if other inventors have filed patent applications that are considered to be prior art.
Now what I find fascinating here is that no other (pseudo)halogens at the 2 position and ONLY the 5-methoxy and 5-hydroxy derivatives are covered. In addition, only the 2-cyclohexanone ring and the N-methyl substitution are covered. Furthermore, Parke-Davis is most assuredly a US-based company but as far as I can tell, they only applied for (and were granted) a Great Britain patent. Now MAYBE GB patents were cheaper, maybe a preceding US patent prevented them pursuing a patent there... but the two things combined strongly suggest that the inventors believed they had something special but didn't want to make their competitors aware of their developments?
It's worth noting that I've read around 120 patents referring to the arylcyclohexylamine (and bioisosteres) class and I don't think anywhere covered any ortho substituent than -Cl & -OCH3. I don't know why but I have made a significant (for me) amount of money by finding 'holes' in an existing patent thus allowing a patent-holder to be faced with what is known in the trade as a 'me too' drug. It's common practice. So quite why Parke-Davis didn't cover the -F,-CF3,-CN,-Br and so on isn't quite clear.
Anyone who has been through the process of applying for a patent for a new drug will appreciate the cost and effort involved and so the tendency is to attempt to ensure every possible homologue is covered. Now to be clear, you cannot patent what you cannot make but the N-ethyl homologue of PCP (PCE) AKA CI-400 was known (based on it's code number) and indeed is covered by the original patent for PCP (US 3097136). But this later patent for a ketamine derivative... nothing. Was their a cost limitation (seems unlikely since patents are costly) or was their a time limitation/risk (disubstitution certainly wasn't covered in earlier patents)?
The top link is obviously the subject of the thread but that second link it worth anyone who reads journals concerning drug discovery may well find useful. It lists the letter codes used by the various companies so when faced with something like 'Ro4-1539' you will immediately know it's Roche lab 4.
But I include if for a more specific reason.
Cl-398 phencyclidine
CI-400 eticyclidine
CI-581 is ketamine.
CI-634 is tiletamine.
CL-966 is tenocyclidine
GK-11/OTO-313 is gacyclidine
That last one is most likely Glaxo Kline who sold the rights to Otonomy.
US Patent 5972952 'Neuroprotective pharmaceutical composition containing stereoisomers of arylcyclohexylamines' and this paper basically explores the various chiral side-chains on the cyclohexane moiety of PCP & TCP:

Évolution conformationnelle de la phencyclidine (PCP) et de son analogue thiénylé (TCP) en fonction du milieu
On démontre par 13C RMN, que dans des solvants oxygénés anhydres, non miscibles à l'eau, la position de l'équilibre conformationnel de la phencyclidin…
I HAVE read it (long ago) and most certainly some of the enantiomers studied were significantly more potent than the parent produuct.

Effect of lowered lipophilicity on the affinity of PCP analogues for the PCP receptor and the dopamine transporter
Oxygen and sulphur atoms were introduced in the cyclohcxyl and piperidinyl moieties of the basic structures 1-(1-phenyl-cyclohexyl)piperidine (PCP), 1…
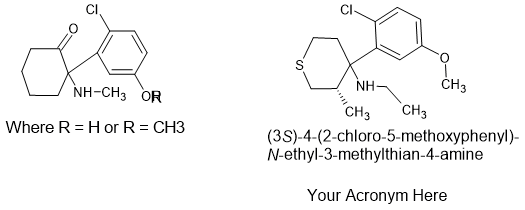
And finally it was discovered that replacing the cyclohexane ring with a 4-thiane ring dramatically increased activity, was compatible with an ortho methyl side-chain.
This last point is likely of most practical use to anyone seeking to research the arylcyclohexylamine class of NMDA antagonist as even nations that employ Markush structural definitions to control analogues do not appear to have considered that the cyclohexane moiety COULD be replaced. The 4-oxane analogues were also explored and while active, were significantly less so than the thianes. A useful 'Plan B'.
Just to be clear, gacyclidine wasn't the most potent homologue but when designing a medicine, many factors other than potency are important and I suspect that reducing the DRI activity would be one detail and researcher would consider when choosing a candidate. The goals of designing a medicine and a pseudohallucinogen don't always match up.
The Ketamine Analogue Methoxetamine and 3- and 4-Methoxy Analogues of Phencyclidine Are High Affinity and Selective Ligands for the Glutamate NMDA Receptor - PMC
In this paper we determined the pharmacological profiles of novel ketamine and phencyclidine analogues currently used as ‘designer drugs’ and compared them to the parent substances via the resources of the National Institute of Mental Health ...
The above paper states the Ki of 3-MeO PCP to be 20 and MK-801 to be 4.8 but if memory serves, at least one of the 4-thianes had an even higher affinity. This is me searching my memory from a decade ago but I used ChemOffice and several other sources to calculate the LogP and other relevant physical properties and estimated that sub-milligram doses should be active.
Now Reaxys DID recognize the furan analogue of TCP but no references were supplied.
I have mentioned this before but I felt it worth starting a thread as the QSAR of the arylcyclohexylamines has been studied for over 30 years and yet RC manufacturers appear to be just guessing.
Finally, the person who designed MXE (still my pick of the ACA class of pseudohallucinogens did politely remark that the receptor appears to have sufficient space for an ETHOXY moiety so EXE would, I imagine, be the simplest new analogue to try.
I'm sure both of us would be delighted to field test any novel analogues.




