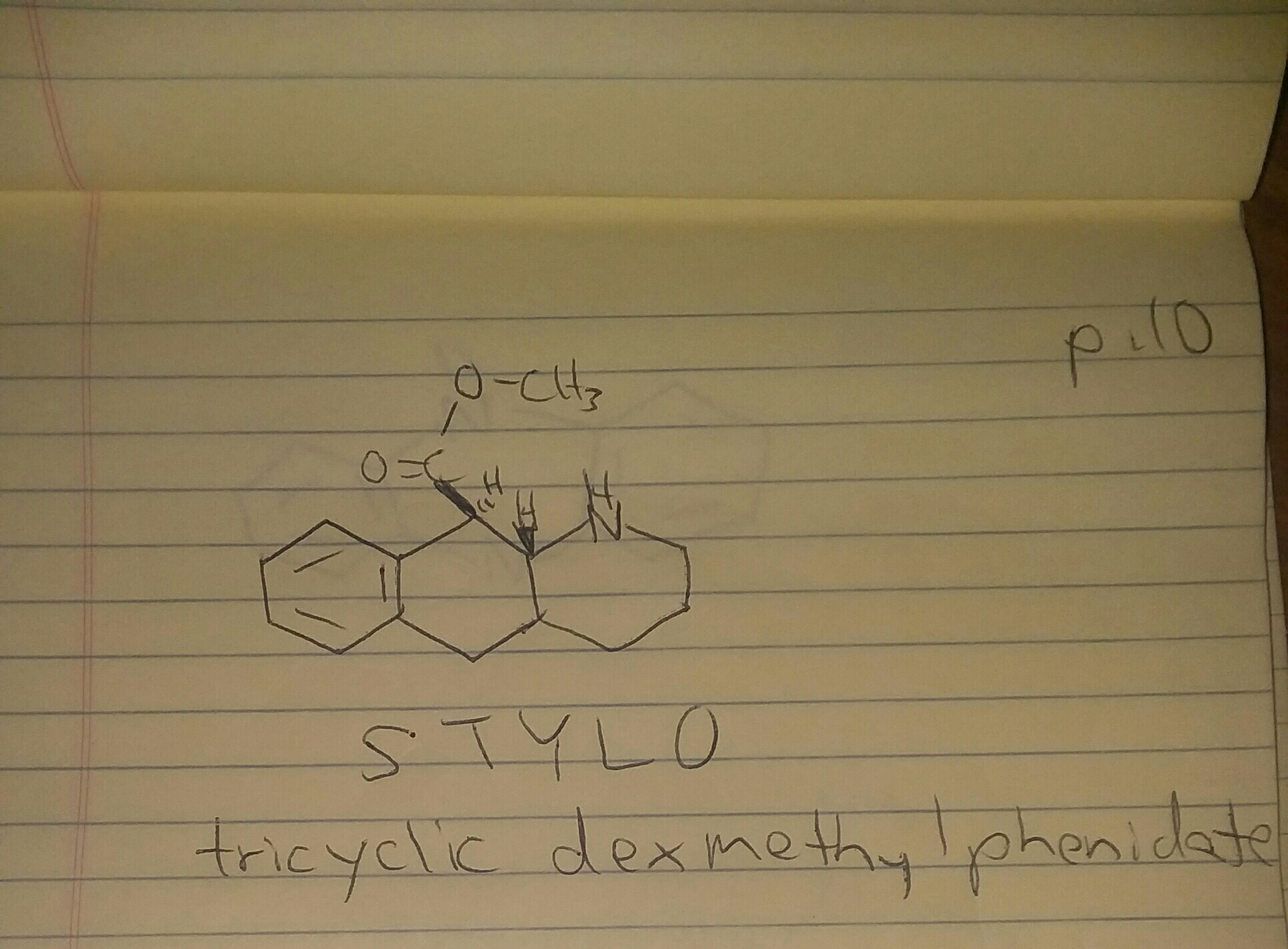
-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Ketamine salts solubility
- Thread starter fastandbulbous
- Start date
- Status
- Not open for further replies.
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,484
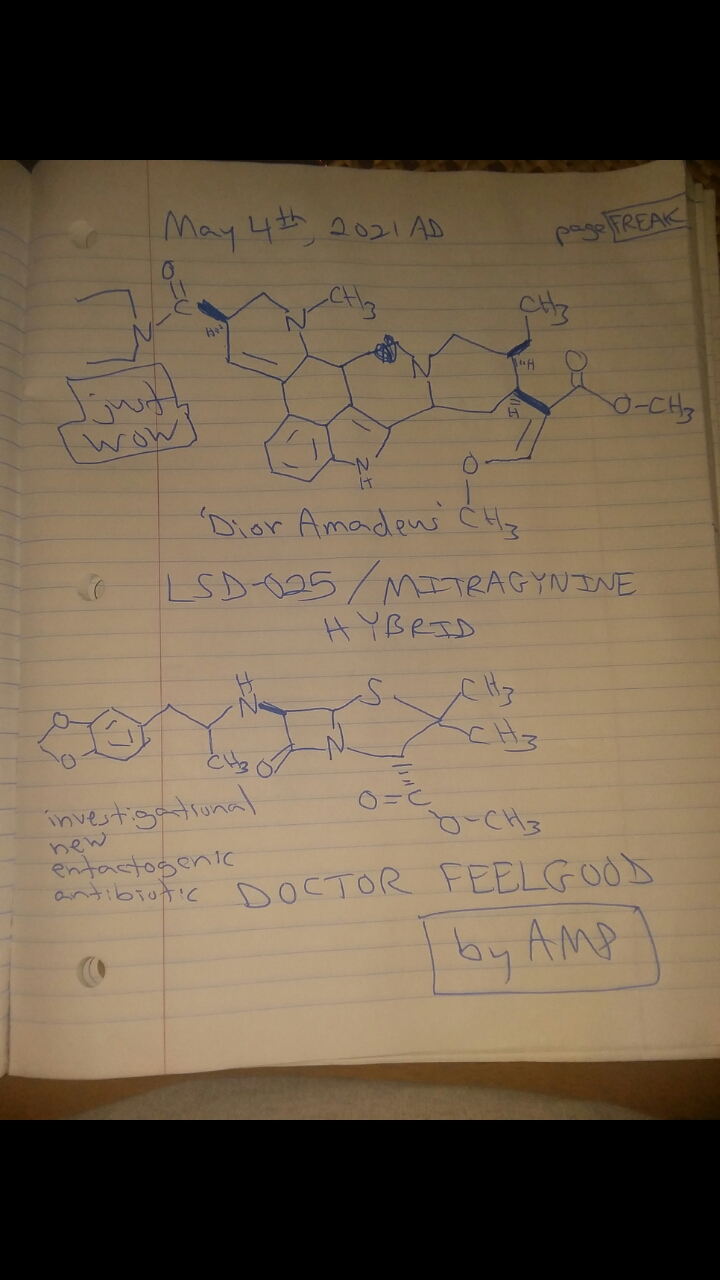
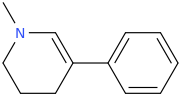
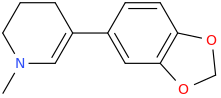
The LSD-025/mitragynine hybrid I posted the other day and colloquially named, 'Dior Amadeus,' has a super interesting property: Namely, it goes from being a mydriatic stimulant to a miosis producing opiate roughly 12 (6 to 24) hours later.
The reason for this property is that the piperidine ring with one double bond probably gets metabolically aromatized by the body's aromatase enzyme to a toxic pyridinium species whose cationic nitrogen no longer supports the stimulatory activity brought about by the methamphetamine pharmacophore found in the structures of both LSD-025 and Dior Amadeus.
Additionally, the nitrogen atom found in the mitragynine portion of Dior Amadeus still activates the strong opioid activity (pin point pupils, for example) noted a day or two into a Dior Amadeus session.
Fascinating!
The reason for this property is that the piperidine ring with one double bond probably gets metabolically aromatized by the body's aromatase enzyme to a toxic pyridinium species whose cationic nitrogen no longer supports the stimulatory activity brought about by the methamphetamine pharmacophore found in the structures of both LSD-025 and Dior Amadeus.
Additionally, the nitrogen atom found in the mitragynine portion of Dior Amadeus still activates the strong opioid activity (pin point pupils, for example) noted a day or two into a Dior Amadeus session.
Fascinating!
izo
Bluelighter
- Joined
- Mar 22, 2006
- Messages
- 4,165
New analgesic drugs derived from phencyclidine
Several esters of 1-(l -phenylcyclohexyl)-4-piperidinol(3)1, -(l -phenylcyclohexyl)-4-phenyl-4-piperidin(1o0l) and
its propionate (1l ),a nd 1-(l -phenylcyclohexyl)-4-phenylpiperidine(1 3) were prepared and characterized. The new
compounds, which are derived from phencyclidine, exerted analgesic activity in mice. The most potent is 10, which
is twice as active as morphine. The antinociceptive activity of 10, 11, and 13 could be well correlated with their
potency in the mouse vas deferens bioassay, and both were completely reversed by naloxone.
they made some pethidine type pcp hybrids linked together at the piperidine moiety.

New analgesic drugs derived from phencyclidine - PubMed
Several esters of 1-(1-phenylcyclohexyl)-4-piperidinol (3), 1-(1-phenylcyclohexyl)-4-phenyl-4-piperidinol (10) and its propionate (11), and 1-(1-phenylcyclohexyl)-4-phenylpiperidine (13) were prepared and characterized. The new compounds, which are derived from phencyclidine, exerted analgesic...
1981 - new_analgesic_drugs_derived_from_phenycylidine.pdf
 drive.google.com
drive.google.com
Last edited:
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
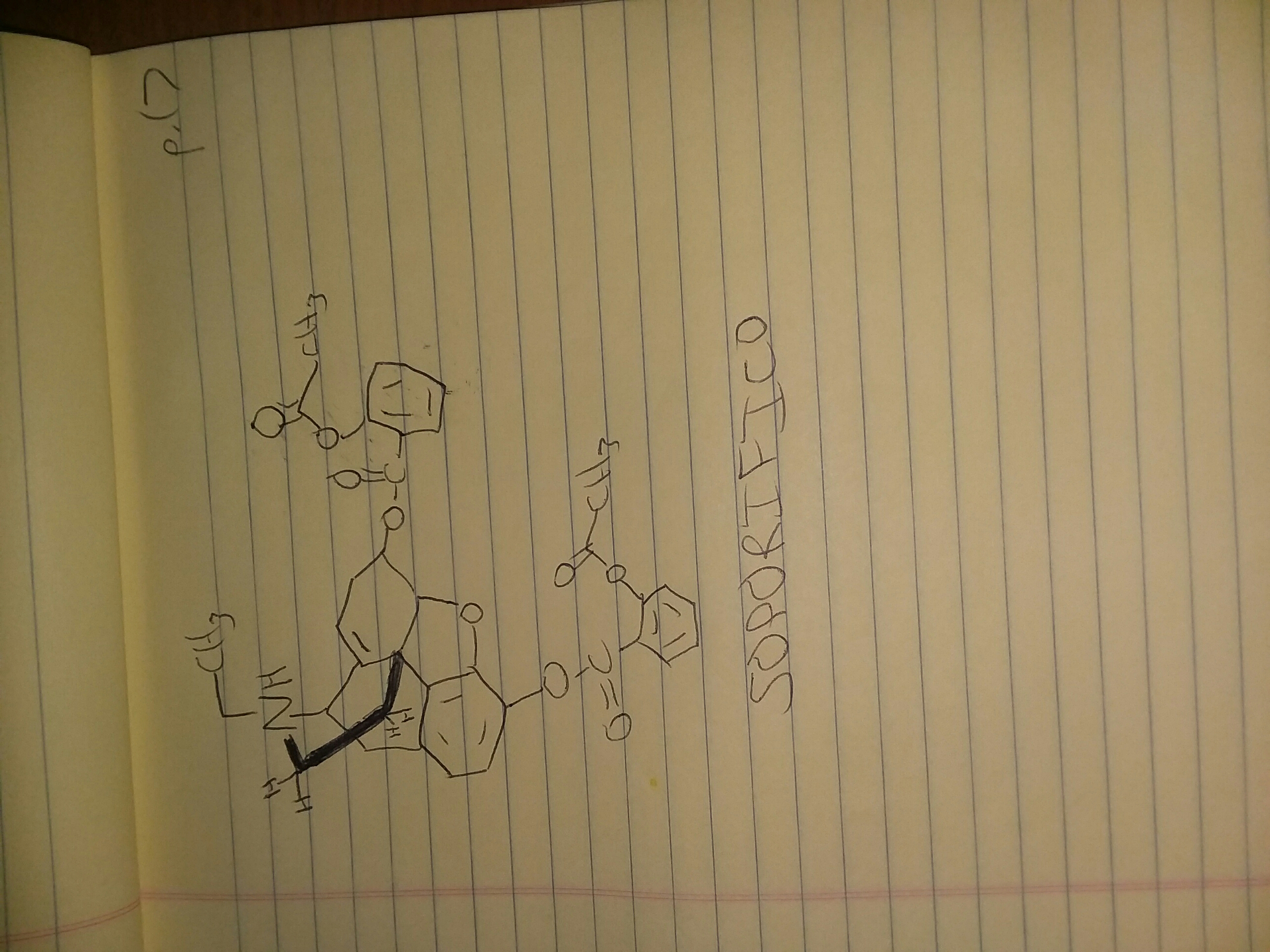
I liké Connie, nice one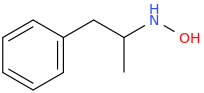
CHRISTA
1-phenyl-2-(hydroxyamino)propane
SAMMY
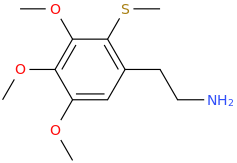
1-(2-methylthio-3,4,5-trimethoxyphenyl)-2-aminoethane
CONNIE
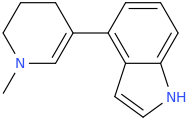
1-methyl-3-(indole-4-yl)-1-azacyclohex-2-ene
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,484
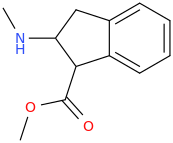
NURSE BETTY
2-methylamino-3-carbomethoxyindan
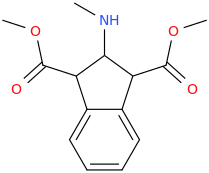
HYPER_ACTIVE
2-methylamino-1,3-dicarbomethoxyindan
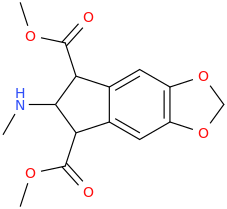
HENRY
2-methylamino-1,3-dicarbomethotxy-5,6-methylenedioxyindan
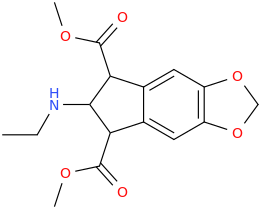
JESSICA
2-ethylamino-1,3-dicarbomethoxy-5,6-methylenedioxyindan
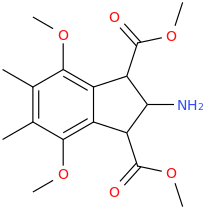
HONEY BEAR
2-amino-1,3-dicarbomethoxy-4,7-dimethoxy-5,6-dimethylindan
Last edited:
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
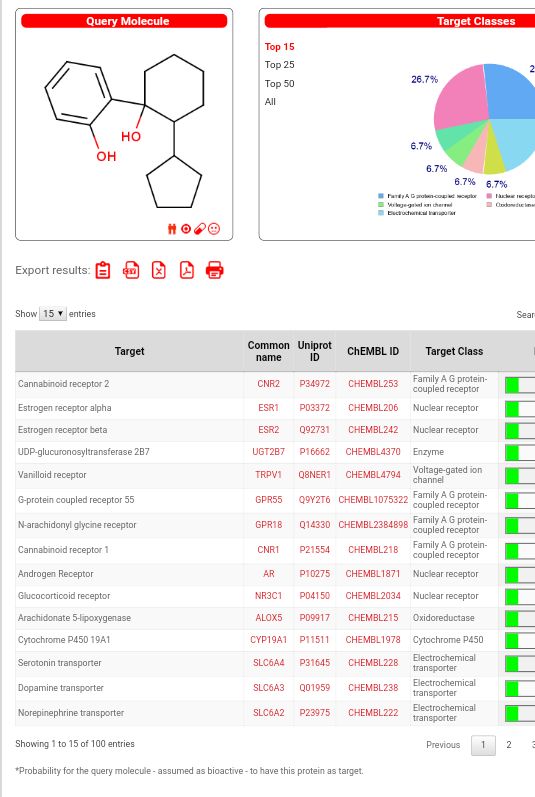
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
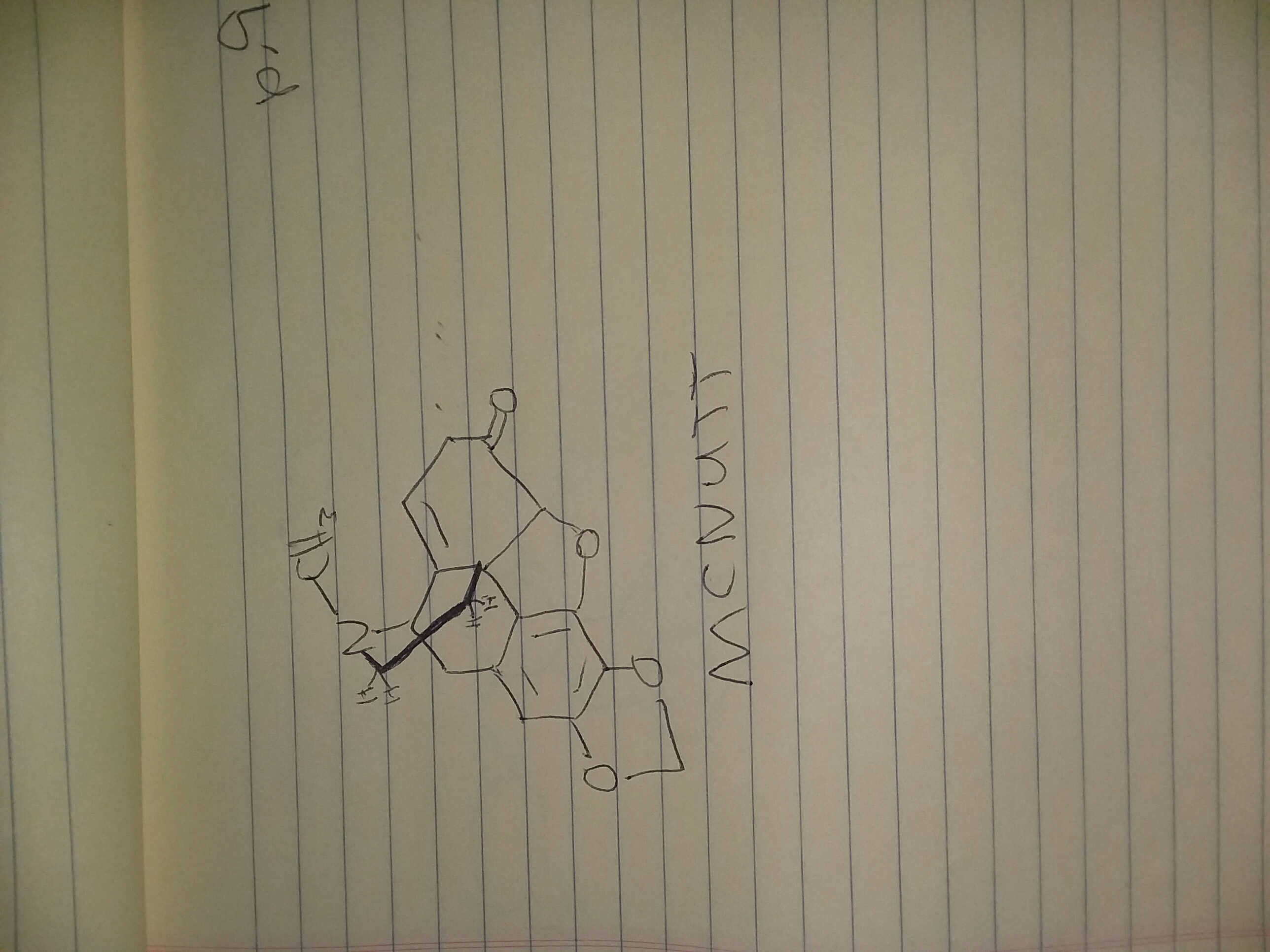
Nurse Betty could bé good

NURSE BETTY
2-methylamino-3-carbomethoxyindan

HYPER_ACTIVE
2-methylamino-1,3-dicarbomethoxyindan

HENRY
2-methylamino-1,3-dicarbomethotxy-5,6-methylenedioxyindan

JESSICA
2-ethylamino-1,3-dicarbomethoxy-5,6-methylenedioxyindan

HONEY BEAR
2-amino-1,3-dicarbomethoxy-4,7-dimethoxy-5,6-dimethylindan
izo
Bluelighter
- Joined
- Mar 22, 2006
- Messages
- 4,165
Behavioral and serotonin receptor properties of 4-substituted derivatives of the hallucinogen 1-(2,5-dimethoxyphenyl)-2-aminopropane
Abstract
The serotonin (5-HT) receptor affinities and behavioral (discriminative stimulus) properties of a series of 4-substituted derivatives of 1-(2,5-dimethoxyphenyl)-2-aminopropanes (2,5-DMA) were investigated. The substituents at the 4-position included H, OMe, OEt, Me, Et, F, Br, I, and NO2. Substituent lipophilicities (pi values) of these functionalities appear to have a minimal effect on either 5-HT receptor affinity or behavioral activity. Those derivatives previously found to be most potent in human studies possess significant affinity for 5-HT receptors. Furthermore, when rats trained to discriminate (+/-)-1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM) from saline were used, generalization was found to occur upon administration of the 4-substituted 2,5-DMA derivatives. Because a direct relationship exists between the ED50 values obtained from these discrimination studies and human hallucinogenic potencies, the discriminative stimulus paradigm, with DOM as a training drug, appears to be a useful tool for comparing the quantitative and qualitative (DOM-like) effects produced by certain hallucinogenic agents.

Behavioral and serotonin receptor properties of 4-substituted derivatives of the hallucinogen 1-(2,5-dimethoxyphenyl)-2-aminopropane - PubMed
The serotonin (5-HT) receptor affinities and behavioral (discriminative stimulus) properties of a series of 4-substituted derivatives of 1-(2,5-dimethoxyphenyl)-2-aminopropanes (2,5-DMA) were investigated. The substituents at the 4-position included H, OMe, OEt, Me, Et, F, Br, I, and NO2...
Last edited:
- Status
- Not open for further replies.