-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Ketamine salts solubility
- Thread starter fastandbulbous
- Start date
- Status
- Not open for further replies.
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
Out of all possible configurations of a xanthine, when a simple methylene unit added will do?Not metabolically likely at all.
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,484
Methylene is R-CH2-R', where R stands for radical.
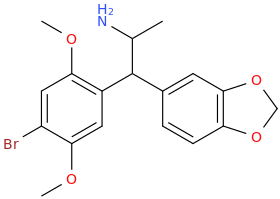
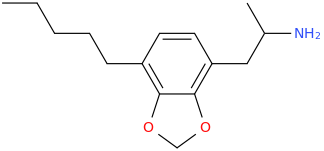
ROEROE
1-(4-bromo-2,5-dimethoxyphenyl)-1-(3,4-methylenedioxyphenyl)-2-aminopropane
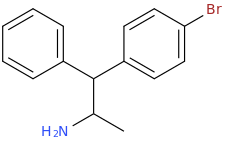
HOPE
1-phenyl-1-(4-bromophenyl)-2-aminopropane
CHAD
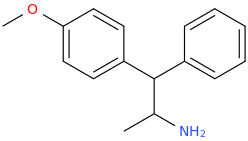
1-(4-methoxyphenyl)-1-phenyl-2-aminopropane
TROY
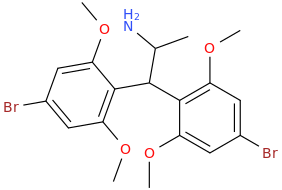
1,1-bis-(2,6-dimethoxy-4-bromophenyl)-2-aminopropane

ROEROE
1-(4-bromo-2,5-dimethoxyphenyl)-1-(3,4-methylenedioxyphenyl)-2-aminopropane

HOPE
1-phenyl-1-(4-bromophenyl)-2-aminopropane

CHAD
1-(4-methoxyphenyl)-1-phenyl-2-aminopropane

TROY
1,1-bis-(2,6-dimethoxy-4-bromophenyl)-2-aminopropane
Last edited:
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
I meant any substitution coming off the nitrogen that eventually ended in one, not straight off of it.Methylene is R-CH2-R', where R stands for radical
Regardless, I wasn't trying to get in a debate about the viability, and which is why I did not limit the scope and present as example one specific instance of any sort, partly due to knowing that making it active would be a chore. I just thought the concept would make for a witty, facetious, name.
Namely (pun intended) It was a purposely abstract and flippant (much as this whole thread) request, not an invitation for analysis. ;-P I can't help but feel this thread needs more substance, setting any kind of precedent in that regard was my intention, broaden the spectrum of content a bit with some interaction, have something more than a series of stand alone posts.
- Joined
- May 11, 2011
- Messages
- 3,412
Did I Lie To You?
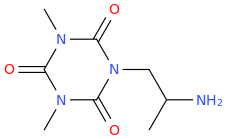
MAGIC POISON
1-(2,4,6-trioxo-1,3,5-triaza-3,5-dimethylcyclohexane-1-yl)-2-aminopropane
Life Of
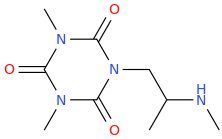
BRIAN
1-(2,4,6-trioxo-1,3,5-triaza-3,5-dimethylcyclohexane-1-yl)-2-(methylamino)propane
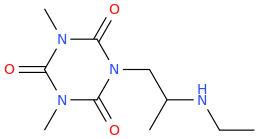
PALLAS 'SCARLET' ATHENA de Swainsboro
1-(2,4,6-trioxo-1,3,5-triaza-3,5-dimethylcyclohexane-1-yl)-2-(ethylamino)propane
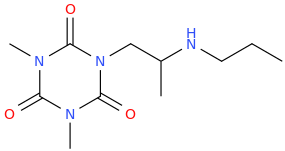
CHRISTOPHER 'DINO' DANIEL BOWEN
1-(2,4,6-trioxo-1,3,5-triaza-3,5-dimethylcyclohexane-1-yl)-2-(propylamino)propane
Bon apetit!
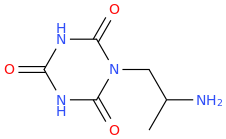
MONA
1-(2,4,6-trioxo-1,3,5-triazacyclohexane-1-yl)-2-aminopropane
These all look like disinfectants.
- Joined
- May 11, 2011
- Messages
- 3,412
That's getting too close to busulfan for my tastes.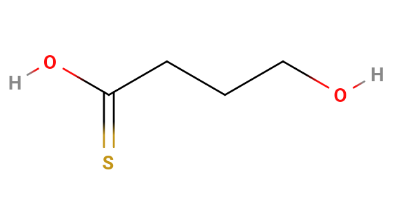
“Green Vertigo” (GHB with a sulphur substitution at the oxygen double bond; though I'm assuming the sulphur would do something like pick up oxygens. Play on "vert", heraldic French for 'green', also latin for "turn")
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
Well now, then the name is more suitable than I first figured; turns you green and gives you vertigo. (I was simply basing it on my only GHB experience.)That's getting too close to busulfan for my tastes.
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,484
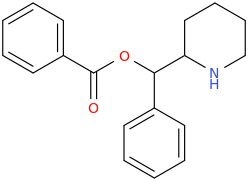
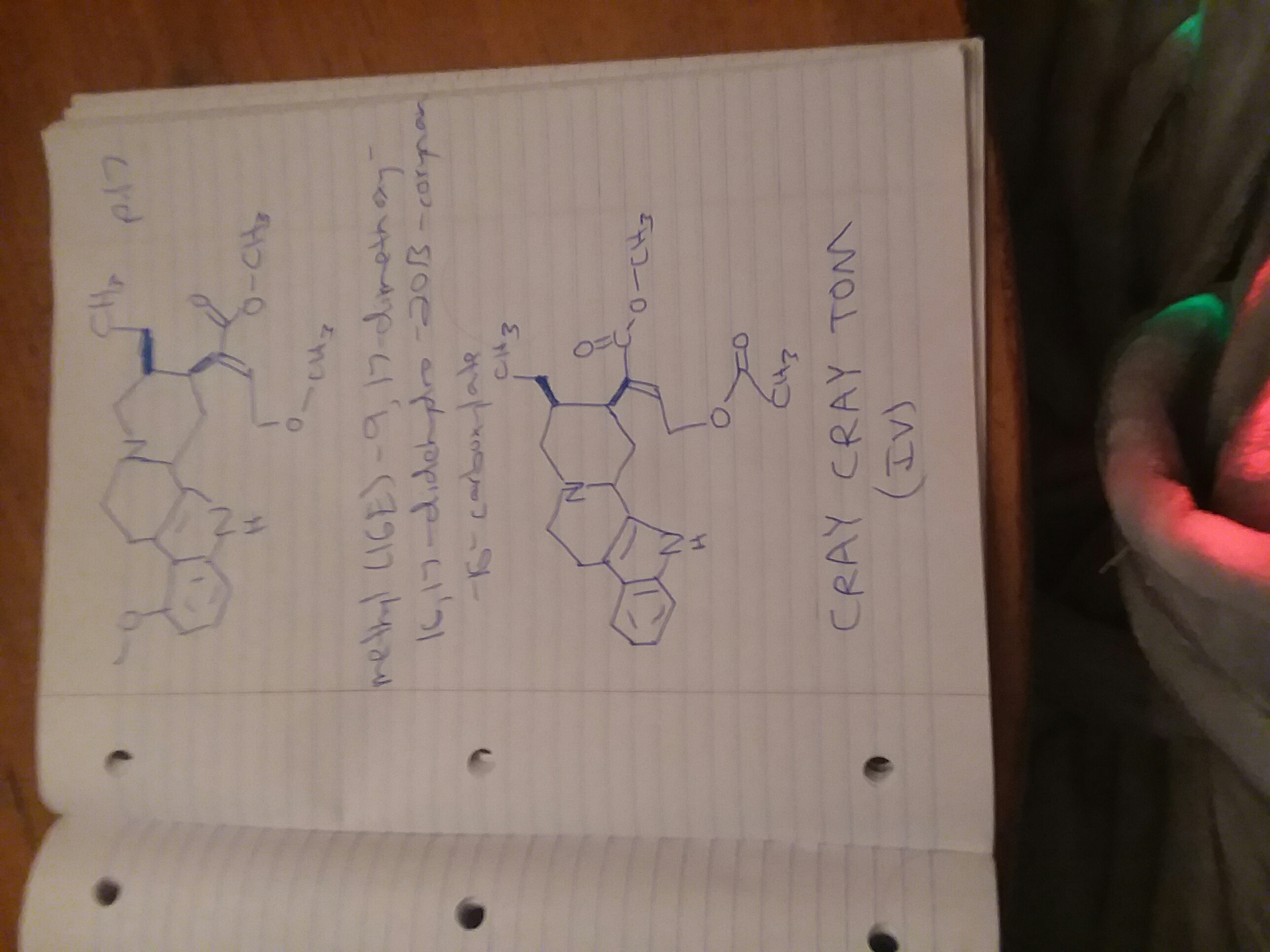
SAINT PETER
1-phenyl-1-(2-piperidinyl)-1-(phenylcarbonyloxy)methane
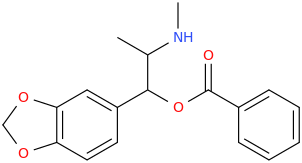
MAYA
1-(3,4-methylenedioxyphenyl)-1-(phenylcarbonyloxy)-2-methylaminopropane
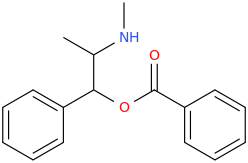
YAMA
1-phenyl-1-(phenylcarbonyloxy)-2-methylaminopropane
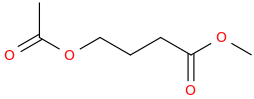
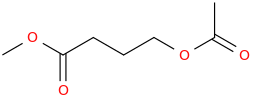
SWIRL (GHB PRO DRUG)
1-acetoxy-3-carbomethoxypropane
Last edited:
- Joined
- May 11, 2011
- Messages
- 3,412
Antidepressant drugs act by directly binding to TRKB neurotrophin receptors
Plinio C. Casarotto
Mykhailo Girych
Senem M. Fred
Mart Saarma
Ilpo Vattulainen
Eero Castrén 15, 17
Show all authors
Show footnotesOpen AccessPublished:February 18, 2021DOI:https://doi.org/10.1016/j.cell.2021.01.034
PlumX Metrics
Highlights
•
Several antidepressants, including SSRIs and ketamine, directly bind to TRKB
•
TRKB dimerization at transmembrane region forms a binding pocket for fluoxetine
•
Antidepressant binding to TRKB facilitates BDNF action and plasticity
•
Point mutation in TRKB transmembrane region blocks the effects of antidepressants
Summary
It is unclear how binding of antidepressant drugs to their targets gives rise to the clinical antidepressant effect. We discovered that the transmembrane domain of tyrosine kinase receptor 2 (TRKB), the brain-derived neurotrophic factor (BDNF) receptor that promotes neuronal plasticity and antidepressant responses, has a cholesterol-sensing function that mediates synaptic effects of cholesterol. We then found that both typical and fast-acting antidepressants directly bind to TRKB, thereby facilitating synaptic localization of TRKB and its activation by BDNF. Extensive computational approaches including atomistic molecular dynamics simulations revealed a binding site at the transmembrane region of TRKB dimers. Mutation of the TRKB antidepressant-binding motif impaired cellular, behavioral, and plasticity-promoting responses to antidepressants in vitro and in vivo. We suggest that binding to TRKB and allosteric facilitation of BDNF signaling is the common mechanism for antidepressant action, which may explain why typical antidepressants act slowly and how molecular effects of antidepressants are translated into clinical mood recovery.
There is a new explanation of antidepressant mechanism of action in Cell. Basicly many antidepressants have been found to bind at low affinity to a region of Trkb receptors (these are activated by BDNF).
They have a good explanation for why ssris take a long time to work even when through the same proposed mechanism as rapid acting drugs like ketamine, which is that the Trkb binding is low affinity and requires concentrations in the brain to reach relatively high steady state levels of drug.
The paper actually has a pretty big focus on how the functioning of the Trkb receptor involves monitoring cholesterol levels on the neuron. It touches on correlations between brain cholesterol and psychiatric illness.

Plinio C. Casarotto
Mykhailo Girych
Senem M. Fred
Mart Saarma
Ilpo Vattulainen
Eero Castrén 15, 17
Show all authors
Show footnotesOpen AccessPublished:February 18, 2021DOI:https://doi.org/10.1016/j.cell.2021.01.034
PlumX Metrics
Highlights
•
Several antidepressants, including SSRIs and ketamine, directly bind to TRKB
•
TRKB dimerization at transmembrane region forms a binding pocket for fluoxetine
•
Antidepressant binding to TRKB facilitates BDNF action and plasticity
•
Point mutation in TRKB transmembrane region blocks the effects of antidepressants
Summary
It is unclear how binding of antidepressant drugs to their targets gives rise to the clinical antidepressant effect. We discovered that the transmembrane domain of tyrosine kinase receptor 2 (TRKB), the brain-derived neurotrophic factor (BDNF) receptor that promotes neuronal plasticity and antidepressant responses, has a cholesterol-sensing function that mediates synaptic effects of cholesterol. We then found that both typical and fast-acting antidepressants directly bind to TRKB, thereby facilitating synaptic localization of TRKB and its activation by BDNF. Extensive computational approaches including atomistic molecular dynamics simulations revealed a binding site at the transmembrane region of TRKB dimers. Mutation of the TRKB antidepressant-binding motif impaired cellular, behavioral, and plasticity-promoting responses to antidepressants in vitro and in vivo. We suggest that binding to TRKB and allosteric facilitation of BDNF signaling is the common mechanism for antidepressant action, which may explain why typical antidepressants act slowly and how molecular effects of antidepressants are translated into clinical mood recovery.
There is a new explanation of antidepressant mechanism of action in Cell. Basicly many antidepressants have been found to bind at low affinity to a region of Trkb receptors (these are activated by BDNF).
They have a good explanation for why ssris take a long time to work even when through the same proposed mechanism as rapid acting drugs like ketamine, which is that the Trkb binding is low affinity and requires concentrations in the brain to reach relatively high steady state levels of drug.
The paper actually has a pretty big focus on how the functioning of the Trkb receptor involves monitoring cholesterol levels on the neuron. It touches on correlations between brain cholesterol and psychiatric illness.

Antidepressant drugs act by directly binding to TRKB neurotrophin receptors
Direct binding of both typical and fast-acting antidepressants to the BDNF receptor TRKB accounts for cell biological and behavioral actions of antidepressants. This mechanism directly connects antidepressant action to neuronal plasticity and may explain the slow action of typical antidepressants.
www.cell.com
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
Inhaludon. (phenmetrazine ‘preludin’ + nicotine)
I was entertaining the possibility of presenting a simple molecular part, and someone else taking that as a base and adding a functional group (with heteroatoms intact, etc.), and either going back to the OP for a final addition of a branch off from that, or a third party finishing it. Would we come up with anything potentially active? Almost a form of tic-tac-toe (Were our contributions at odds with what the other intended to achieve)
Then we could each give a speculative name of the end product, rather than what we were trying to make or the concept we may have initially had but didn't reach fruition.
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,484
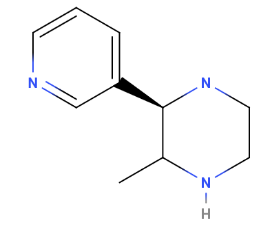
Ok, but first let me post one that I had just thought of before reading your post.
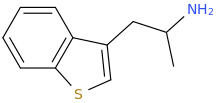
DAWN
1-(benzothiophene-3-yl)-2-aminopropane
Last time I did AMT I really can't say that I enjoyed it, but Dawn is probably completely different based on Nichols' finding that dimethylaminoethylbenzothiophene (DMT with a sulfur for the heterocycle) is inactive. Therefore, Dawn should act more like a pea than a tryptamine.

DAWN
1-(benzothiophene-3-yl)-2-aminopropane
Last time I did AMT I really can't say that I enjoyed it, but Dawn is probably completely different based on Nichols' finding that dimethylaminoethylbenzothiophene (DMT with a sulfur for the heterocycle) is inactive. Therefore, Dawn should act more like a pea than a tryptamine.
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,484
Ok, so I'm back. I made a couple o changes to your molecule and named it...
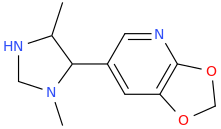
LOVE'S
1-methyl-2-(3,4-methylenedioxy-5-azaphenyl)-3-methyl-1,4-diazacyclopentane
I'm hoping it will be lovely.
Of course, due to chirality (the molecule has 2 stereo center carbons as drawn), we are really looking at 4 molecules (2^n) here.

LOVE'S
1-methyl-2-(3,4-methylenedioxy-5-azaphenyl)-3-methyl-1,4-diazacyclopentane
I'm hoping it will be lovely.
Of course, due to chirality (the molecule has 2 stereo center carbons as drawn), we are really looking at 4 molecules (2^n) here.
- Status
- Not open for further replies.