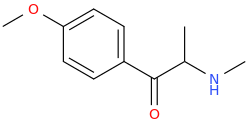
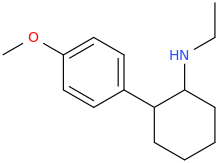
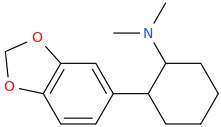
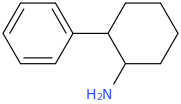
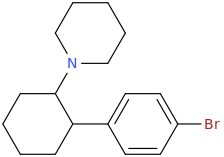
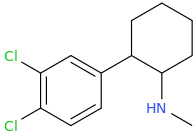
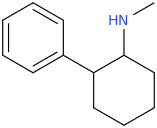
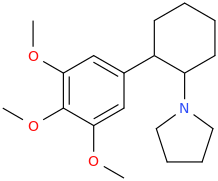
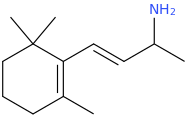
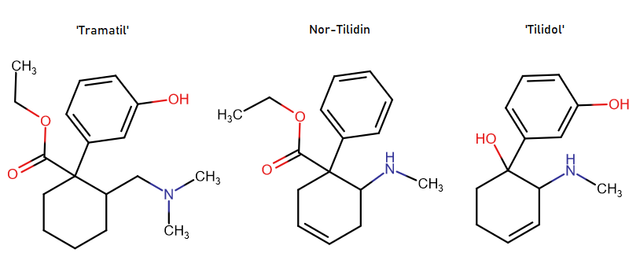
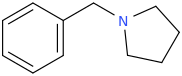
Yes I thought about a halogenated version but I wonder why it hasn't been considered by the big RC industry yet? Is desmethyl-Tramadol in general somewhat tricky or pricey to synthesize? Otherwise I see no reason why there haven't been many analogues already popping up.
But somehow I'm not a big fan of halogenated compounds anyways, besides benzodiazepines. Maybe because of my experiences with synthetic cannabinoids, if you smoke so many different compounds you somehow get a "feeling" for what makes fluorinated noids different in general, not that it's feeling or being more toxic or so, I can't find words for what I want to say. Maybe it has to do with the western pharmaceutical system where everything gets halogenated so you can sell 1/100 the amount and quadruple the profit, just like we have seen with synthetic noids. The more potent the better and mainly because of economics, I wonder how many good compounds there are out there that maybe need bigger doses, don't have the narrowest possible effect spectrum etc. to be patentable but would help much more with less side effects as an addition to the medicine spectrum.
I'm always fascinated by the russian pharma industry during sovjet times, even though they didn't have better or as-good medicine at all, there are so many interesting compounds and general ways of looking on how to make medicine, from the natural extracts like Schizandra, Eleuthero and Rhodiola to compounds like Phenibut or Picamilon, the racetams like Phenylpiracetam or Noopept and the peptide compounds like Semax of which there have been thousands being researched. Maybe sometimes it doesn't need a high potent patentable compound when glueing niacine and gaba together is enough to get a cheap and effective medicine for mild conditions with no side effects. A bit like a bridge between natural and chemical therapy.
But I'm getting off topic here...
If the first compound would convert into clonazepam inside the body by ring-closure, would the second compound create Meclonazepam (not Methylclonazepam)? I loved that stuff, favorite benzo after Etizolam.
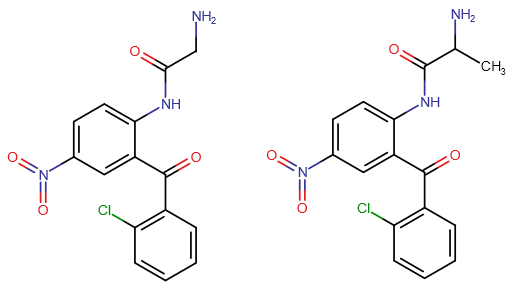
How dangerous do you think are those benzophenone-prodrugs? For me as a layman they look a bit suspect with those two benzol rings, but it seems like a great way to play around with many new prodrugs. Established ones like pro-Diazepam are known to be safe even when injected into the muscle or blood, and from what I found in literature others like Alpra-Triazolobenzophenone shouldn't be very toxic, but for example I couldn't find one that uses the nitro unit in such a pro-drug, or the pyridine ring like Bromazepam and Pyrazolam. Could those impart toxic properties, or haven't they been considered because of lower effect possibility, worse perfomance, etc.?