-
Select Your Topic Then Scroll Down
Alcohol Bupe Benzos Cocaine Heroin Opioids RCs Stimulants Misc Harm Reduction All Topics Gabapentinoids Tired of your habit? Struggling to cope?
Want to regain control or get sober?
Visit our Recovery Support Forums -
OD Moderators: Keif’ Richards | negrogesic
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Opioids DiAcetylHydromorphone?
- Thread starter G_Chem
- Start date
Prettyflyforawifi
Greenlighter
- Joined
- Jan 25, 2020
- Messages
- 7
If you take the 4s shoot it. Rock your done. I just ruined an 8 cause it gummed up like Opana
Anyone know much about this or any of the esters of hydromorphone (Dilaudid)?
I can only assume that this is like the god of all opioid rushes but there doesn’t seem to be much info on it compared to diacetyldihydromorphine.
-GC
Making diacetyldihydromorphone might be harder than you'd expect because the molecule only has one hydroxyl group; the 6-hydroxyl group has been converted to a ketone, after all. It is possible to acetylate it in its enolic tautomer form (as is the case with thebacon, or hydrocodone enol acetate) though.
kaosisallwesee
Bluelighter
I was going to say the same thing, you can't make diacetyl or dihydromorphone. Not in the same way as morphine anyway. The ketone at six position prevents it. If you removed that ketone in order to add a second acetyl group it'd just be diacetylmorphine.Making diacetyldihydromorphone might be harder than you'd expect because the molecule only has one hydroxyl group;
sekio
Bluelight Crew
you can actually make the enol ester of a ketone, it just takes a little work, see: thebacon - it is the acetate of hydrocodone, at the ketone position. the 3-acetate of hydromorphone is a known compound too: acetylmorphone
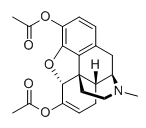
diacetylhydromorphone? or, taking a page from dresden and by analogy to thebacon, maybe thescrambledeggs? ....
didn't william burroughs say tri-acetoxy-oxymorphone would be a junkie's dream?

diacetylhydromorphone? or, taking a page from dresden and by analogy to thebacon, maybe thescrambledeggs? ....
didn't william burroughs say tri-acetoxy-oxymorphone would be a junkie's dream?
Last edited:
didn't william burroughs say tri-acetoxy-oxymorphone would be a junkie's dream?
I believe he called his dream drug "dihydro-oxy-heroin".
Dihydro-oxy-morphine would be oxymorphol (the reduced metabolite of oxymorphone), though the question is whether the "heroin" part refers to the 3,6 diacetyl version, or the fully acetylated 3,6,14 triacetyl oxymorphol.
Also, wouldn't "tri-acetoxy-oxymorphone" be a peroxide? "acetoxy" already includes both the acyl carbonyl and the single-bonded oxygen; adding an acetoxy group to a substance that already has hydroxyl oxygens (like morphine/oxymorphone) would make it an organic peroxide (hence why psilacetin is either written as 4-AcO-DMT or O-acetyl-psilocin, but not AcO-psilocin).
Thank you guys, after posting this I did further research to reveal what ya’ll been saying in regards to only being able to produce the 3-acetyl ester. Guess that’s why it was so hard to find any reference to the di ester lol.
Umm I guess anyone with personal experience with 3-acetyl hydromorphone?
-GC
Umm I guess anyone with personal experience with 3-acetyl hydromorphone?
-GC
Final question, @sekio @Hodor @kaosisallwesee
So obviously one way to obtain said product would be acetylation of hydromorphone, but what about creating hydromorphone from 6-MAM vs morphine? If the acetyl can only attach to the 3 position what would happen in this case? Would 3-acetylmorphone still be the end product?
Thank you!
-GC
So obviously one way to obtain said product would be acetylation of hydromorphone, but what about creating hydromorphone from 6-MAM vs morphine? If the acetyl can only attach to the 3 position what would happen in this case? Would 3-acetylmorphone still be the end product?
Thank you!
-GC
Drag2019
Bluelighter
This is a very interesting read. I'm intrigued to see what type of results you can obtain from these Substances and how you can go about creating them.
sekio
Bluelight Crew
You can't produce hydromorphone from 6-MAM, you would have to hydrolyse the ester at the 6' position to allow you to oxidise that same spot to a ketone. Morphine can be catalytically isomerized into hydromorphone in one step anyway: or a two-step direct synthesis (reduce to dihydromorphine, then oxidise - or vice versa)
You'd have to look hard to find an example of an 'acetoxy' compound that is a peroxide. Usually when someone says 'acetoxy' when referring to derivitizing an alcohol they mean converting OH to OAc. were it a peroxide, it would be 'peroxyacetyl'.
I don't think anyone really messes with 'classical' opioid analogues any more... the chemistry is all quite illegal and you need to start with an opium alkaloid anyway. FWIW, dihydroheroin is effectively indistingusishible from diamorphine - that's all I can tell you.
"acetoxy" already includes both the acyl carbonyl and the single-bonded oxygen;
You'd have to look hard to find an example of an 'acetoxy' compound that is a peroxide. Usually when someone says 'acetoxy' when referring to derivitizing an alcohol they mean converting OH to OAc. were it a peroxide, it would be 'peroxyacetyl'.
I don't think anyone really messes with 'classical' opioid analogues any more... the chemistry is all quite illegal and you need to start with an opium alkaloid anyway. FWIW, dihydroheroin is effectively indistingusishible from diamorphine - that's all I can tell you.
Thank you Sekio, I had a feeling in that but needed someone smarter than I to confirm..
Well someone’s been messing with minute quantities of some interesting ones as of lately.. If 6-mono benzoyl dihydromorphine and plain dihydromorphine are anything to go on, then the dihydro analogs are actually quite different IMO from straight heroin. More stimulating, stronger rush, a different kind of euphoria and analgesia seemingly more head based, and unfortunately much stronger comedown than their non hydrogenated counter parts. Not too much more potent mg/mg but slightly.
It’s that type of opiate high where you’ll wind up cleaning the whole house.
All that said, I prefer the good old regular esters over the dihydro counterparts. The comedown is just too brutal in comparison, I can tell getting addicted and withdrawal from these would be a nightmare.
One final note, just as the research states the analgesic effect of morphine goes along a different mechanism than dihydromorphine. I found this to be true in that they can stack with each other for an increased synergistic analgesic effect, also the dihydro analogs aren’t as effected by Suboxone as morphine/heroin/bzm are..
-GC
Well someone’s been messing with minute quantities of some interesting ones as of lately.. If 6-mono benzoyl dihydromorphine and plain dihydromorphine are anything to go on, then the dihydro analogs are actually quite different IMO from straight heroin. More stimulating, stronger rush, a different kind of euphoria and analgesia seemingly more head based, and unfortunately much stronger comedown than their non hydrogenated counter parts. Not too much more potent mg/mg but slightly.
It’s that type of opiate high where you’ll wind up cleaning the whole house.
All that said, I prefer the good old regular esters over the dihydro counterparts. The comedown is just too brutal in comparison, I can tell getting addicted and withdrawal from these would be a nightmare.
One final note, just as the research states the analgesic effect of morphine goes along a different mechanism than dihydromorphine. I found this to be true in that they can stack with each other for an increased synergistic analgesic effect, also the dihydro analogs aren’t as effected by Suboxone as morphine/heroin/bzm are..
-GC




