
Design, synthesis and biological evaluation of 3-amino-3-phenylpropionamide derivatives as novel μ opioid receptor ligands
3-Amino-3-phenylpropionamide derivatives were produced as small molecule mimics of the cyclic octapeptide octreotide from readily available imine 1Sch…
Some years ago someone posted the link to a related compound that was, if memory serves, a kappa agonist. As people will know, in most cases the difference between a mu & kappa ligand is a single methylene spacer..
I have actually been looking for the paper referring to the kappa ligand BUT if anyone has any references to this or, in an ideal world, a pointer to the patent or link to .PDF of paper would be very much appreciated.
But if I were forced to assign a structure based on memory, it would be:
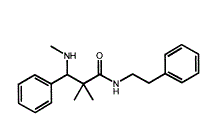
Now, looking at this compound, it has 4 of the key moieties but it is only 14 methylenes long (from the para for the alpha aromatic to para of beta aromatic.
If one overlays the compound with BDPC, one is in for an interesting surprise - both benzene rings & the amine moiety overlay while the -OH moiety of BDPC overlays the =O if the amide function.
The ONLY slight fly in the ointment is that the title compound is only 14 methylenes in length. If one were to borrow from Dan's work, a p-Br (or p-Me) on the alpha benzene ring would seem to be in order.
I cannot find this paper but recall the paper dealing with it's kappa relatives and so I expect phenolic and non-phenolic examples, various chain-lengths and a few other modifications. So why not increase the chain-length? Likely because it increases the number of rotatable bonds BUT I seem to remember that a cinnamyl analogue (3 carbon chaine with an olefinic bond). I am also unable to explain why the tertiary amine was not tried. The synthesis means that the secondary amine would have to be N-methylated and yet I found no examples of this being tested.
So lots of guff - anyone have to paper to check if my ideas are gold or crap?





