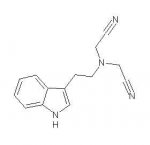
I just thought about a dicyanomethylene tryptamine. It looks a little bit like a cousin of DALT with some exceptions:
A nitrogen at the end of the chain.
A triple bond instead of a double bond.
A straight chain instead of an angulated.
And last but not least a nitrogen atom that is not basic any more.
Can this compound be active?
What would you speculate about its toxicity and possible metabolites?
Has anybody ever seen a cyanomethylene group in any pharm?
A nitrogen at the end of the chain.
A triple bond instead of a double bond.
A straight chain instead of an angulated.
And last but not least a nitrogen atom that is not basic any more.
Can this compound be active?
What would you speculate about its toxicity and possible metabolites?
Has anybody ever seen a cyanomethylene group in any pharm?